R. Martins1, M. Urbich2, K. Brännvall3, M. Gianinazzi3, J.E. Ching4, C.P. Khoury4, Y.H. El-Hayek4
1. Global Market Access Solutions, Health Economics Unit, St-Prex, Switzerland; University of Groningen, University Medical Center Groningen, GZW Global Health department, Groningen, The Netherlands; 2. Biogen International GmbH, Value and Access, HE and HTA Strategy, Switzerland; 3. Biogen International GmbH, Value and Access, Switzerland; 4. Shift Health, Canada
Corresponding Author: Youssef Hanna El-Hayek, 162 Cumberland Street, Suite 310, Toronto Ontario M5R 3N5 Canada, yhayek@shifthealth.com
J Aging Res & Lifestyle 2022;11:38-46
Published online November 15, 2022, http://dx.doi.org/10.14283/jarlife.2022.7
Abstract
Background: Recent advances open the opportunity of altering the course of Alzheimer’s disease (AD) through lifestyle-based modifications and novel therapies. Ensuring that society is investing limited budgets in the interventions that have the greatest
potential to generate tangible impact will require tools to guide policymakers. Objectives: To build on previous studies to develop an economic model that estimates the societal burden of AD and evaluates the potential impact of novel interventions in six large
European countries. Design: AD progression was modelled using a published Markov structure with a 40-year time horizon to
estimate lifetime costs and life years in a cohort aged 65 years and above diagnosed with mild cognitive impairment due to AD (MCI-AD) in 2020. Demographic projections were utilized to estimate the prevalence of MCI-AD up to 2100, total corresponding costs and life years. The model allows a comparison of costs associated with the introduction of a hypothetical new disease-modifying therapy that slows disease progression between MCI-AD and all AD-Dementia stages as well as a ‘delayed onset’ scenario where disease progression is halted at the MCI-AD stage, potentially occurring, for example, through lifestyle-based modifications. Results: The 2022 present value of total lifetime costs for this cohort moving through all disease stages is ~€1.2T. Approximately 80% of the present value of lifetime costs in our model are driven by informal care and non-medical direct costs. Our model suggests that a 25% and 50% reduction in disease progression compared to natural history could translate into a present value of cost savings of €33.7B and €72.7B. Halting MCI-AD progression for 3 years with no therapeutic effect thereafter resulted in a present value cost savings of €84.7B in
savings. Conclusions: Our data further suggest that early intervention via disease-modifying therapies or lifestyle-based
modifications in AD could result in cost savings for society. Additionally, our findings reinforce the importance of accounting for the full value of innovative interventions, management and care paradigms, including their potential impact on direct, indirect and intangible costs impacting patients, their care partners and health and social care systems.
Key words: Alzheimer’s disease, cost of illness, economic model, Europe.
Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder that is increasingly understood in terms of a pathologic continuum with symptoms, impact on quality of life, and overall burden progressing at each stage of the disease (1-3). After a long asymptomatic preclinical period, patients enter a mild cognitive impairment due to AD (MCI-AD) stage, characterized by mild but noticeable issues with memory and thinking. The disease then progresses through a mild stage of dementia, where patients will also exhibit severe lapses in memory, mild language problems and social withdrawal. As patients progress through the moderate stage of dementia, they begin to forget their own personal history and struggle with communication, orientation, sleep and performing simple activities of daily living (ADLs), such as toileting and bathing. By the severe stage of the disease, patients have limited awareness of their surroundings and often lose their ability to speak, walk, sit and even swallow. Each stage of AD intensifies a patient’s level of dependence on health and social care systems, and, most notably, informal care partners, such as family and friends, who are often compelled to make great sacrifices in their personal and professional lives to support their loved ones. Moreover, AD and other forms of dementia are among the top 10 causes of death globally (4).
Current pharmacological treatments for AD only alleviate symptoms and do not address the underlying disease pathology or delay disease progression (5). Nonetheless, several advances are changing the way researchers and care practitioners think about the diagnoses, treatment and management of AD. For example, recent studies have provided a deeper understanding of the modifiable risk factors that could form the basis of lifestyle-based interventions to delay or prevent AD (4, 6-8), as well as the clinical validity and utility of a growing base of biomarkers associated with each stage of the disease (4, 9). Moreover, over 80% of the pharmacological agents in clinical trials are disease-modifying treatments (DMT) that target the underlying biology of AD (10). These potential levers of action create an opportunity to alter the course of AD through prevention, early diagnosis and novel therapies, ultimately alleviating the tremendous burden it creates on patients, their care partners, health and social care systems and wider society. Ensuring that society is investing finite budgets in the interventions that have the greatest potential to generate tangible impact will require tools to guide policymakers.
Several economic models have been developed that have the potential to support AD policy decisions and investments, including those developed by Alzheimer’s Disease International and the Alzheimer’s Association (11-14). Of particular note, Cimler et al. (2019), recently simulated expected AD costs in the EU-28 until 2080, while also assessing the potential impact of the introduction of DMTs at various stages of the AD continuum (14). Similarly, Mukadam et al. (2020) recently evaluated the impact of preventative interventions (targeting hypertension, reducing smoking, providing hearing aids) on costs and dementia prevalence in the UK (15).
Building on this foundational work, we have developed an economic model that quantifies the present value of AD-related care costs and life years in the community and institutional settings across every stage of the disease. We focused on six large European countries with readily available data to estimate a pan-European total. Model results are shown for a base case using no intervention, two scenarios exploring the effect of reducing disease progression between the MCI-AD and AD-Dementia stages using a hypothetical DMT, and a final scenario that estimates the impact of halting disease progression at the MCI-AD stage for 3 years; the latter could be considered to be a hypothetical ‘delayed onset’ scenario, potentially occurring through lifestyle-based modification. Collectively, our analyses aim to explore the potential value of early intervention under different scenarios.
Methods
Model Specification/Mechanics
We have developed an analytical model in Microsoft Excel (Microsoft, Redmond, Washington, US) simulating AD progression in a cohort with prevalent MCI-AD. Alzheimer’s disease progression was modelled using a published Markov structure (16) which allowed the estimation of costs and life years in a cohort of 65 years old individuals, over a 40-year time horizon throughout the full spectrum of the disease in both community and institutional settings. We have expanded this structure using Visual Basic for Application to update the cohort’s starting age in annual increments, up to the age of 100 years, and the sum discounted costs and life years. The resulting estimates are the present value of the lifetime AD-related costs and life years for a cohort of individuals between the ages of 65 and 100 years with prevalent MCI-AD in 2022.
We have utilized publicly available demographic projections for France, Germany, Italy, Spain, Sweden, and the UK from 2022 to 2100 to inform the size of a similar cohort in subsequent annual model iterations (17, 18). The prevalence of mild cognitive impairment (MCI) from any cause was calculated by multiplying the number of individuals in the general population by age specific prevalence figures (19). We assumed that 75% of all cases of MCI were due to AD (20).
The model allows a comparison of costs associated with the current standard of care (SoC) for people with AD to a hypothetical new DMT that slows disease progression between MCI-AD and all AD-Dementia stages by an assumed 25% and 50%, as well as a ‘delayed onset’ scenario where disease progression is halted at the MCI-AD stage for 3 years. We have assumed that this treatment would remain effective in the long-term and that it would not be associated with any treatment discontinuation. AD-Dementia stages include mild, moderate, and severe dementia.
Costs and life years were discounted at a 3% rate after the first annual cycle (21). Published costs were inflated to Euros 2021 using country-specific consumer price indices for health services (22-28) and currency conversion rates (29, 30). Details on the prevalence inputs are available in Table 2 of the Supplemental Materials.
Natural History
All individuals are presented in the model as starting in the MCI-AD state and may transition to the mild, moderate, or severe AD states in subsequent cycles. In the absence of a European publication reporting the probability of progressing from MCI-AD to AD-Dementia and the distribution of progressed people falling in the mild, moderate or severe AD states, data from an analysis of the US National Alzheimer’s Coordinating Center (NACC) was used to inform these model inputs (31). Transitions between AD-Dementia states were informed by an analysis of the Swedish Dementia Registry (32). The authors defined cognitive impairment using Mini-Mental State Examination (MMSE) scores of 21-30 for mild AD, 10 to 20 for moderate AD, and 0 to 9 for severe AD. Transitions back to states of lower AD severity were not assumed to occur.
Institutionalization
The likelihood of becoming institutionalized was sourced from an analysis of NACC data published by Davies and colleagues (33). The prevalence of institutionalization was conditional on AD severity and included an estimate for people at the MCI-AD stage (please see Table 3 in Supplementary Materials).
Mortality
Transition to death was possible from any health state in the model. The annual probability of death in people with AD was calculated by applying the hazard ratios (HR) of MCI-AD (34) or AD-Dementia (35) excess mortality to the annual mortality rates in the general population. Age and gender-specific mortality estimates for the general population were sourced from publicly available life tables for each country (36-41). The inputs utilized to model the natural history of AD, along with their respective sources can be found in Table 3 of the Supplemental Materials.
Treatment Effect
To assess the economic consequences of a hypothetical DMT, a 25% or 50% reduction in transitions between MCI and all AD-Dementia states was applied to the new treatment comparator. This DMT is assumed to be administered continuously over the lifetime time horizon. In a separate scenario, we have also assessed the economic consequences of a lifestyle-based ‘preventive’ strategy that would interrupt MCI-AD progression for 3 years and would have no therapeutic effect thereafter. The scenarios are comparable to those used in other studies such as Cimler et al. 2019 (14).
Costs Literature Search Strategy
A targeted literature search was conducted to identify direct medical, non-medical and informal care costs for all severities of AD for people residing in the community or institutional care settings. We relied on multiple sources including: 1) A review of the references included in recent review papers focused on AD costs (42-44).; 2) the outputs of unpublished systematic literature reviews covering AD cost studies over the period of January 2000 to April 2020; 3) a targeted search of PubMed using search terms related to AD (e.g. “Alzheimer*”, “dementia”, “mild cognitive impairment”) and costs (e.g. “healthcare costs”, “healthcare economics”, “health expenditure”) was also conducted to identify any recent reports published in 2020/2021; 4) general internet searches and reviews of websites of relevant government and non-government organizations for information related to AD costs.
Selection of Cost Inputs and Cost Calculations
After reaching saturation in the targeted literature search, publications providing costs for most cost categories were prioritized for inclusion. If publications reported on the same number of categories, the one with the most robust methodology was selected, with prospective, large-sampled studies being preferred. The exception to the above criteria occurred when selecting the source of informal care costs for Spain. Darbà and colleagues reported costs for community-based individuals at all AD severities (45). Nonetheless, reported costs were 2 to 5 times higher than the similar burden of disease publications in Spain (46, 47). As an example, indirect costs associated with individuals at the severe AD stage were in excess of €105,000 annually, which is substantially above the national average salary in Spain (€44,968) (48) and much higher than the value reported for other European countries in similar publications. We have alternatively assumed that informal care costs in people with MCI-AD were 45% of those for people with mild AD (49). Data were mostly missing for people at the MCI-AD stage, particularly for the informal care costs category. Direct medical (e.g. medication, hospitalization), direct non-medical costs (e.g. community and social services, residential care), and informal care costs (e.g. productivity losses of informal caregivers) associated with individuals with MCI-AD in an institutional care setting were not reported in the literature for any of the European countries included in the analysis. For each country, we have assumed these costs would be identical to the equivalent costs for people with mild AD who were also institutionalized.
Published direct medical costs, direct non-medical costs and informal care costs for people with MCI-AD living in the community were only identified for Spain (45). For the remaining countries, these inputs were derived from the values for people with mild AD in the same country and cost category. These values were adjusted for MCI-AD by extrapolating from a study by Robinson and colleagues who reported that people with MCI-AD had 14.75%, 45.96% and 54.76% lower direct medical, direct non-medical, and informal care costs than people with mild AD, respectively (49).
Informal care costs associated with institutionalized people with mild AD in Sweden and Spain were extrapolated from Prince et al., 2014 (50). In his publication, Prince reported informal care costs for people with mild AD to be 63.22% lower than for people with moderate AD who were also institutionalized (50).
For France, Germany, and Italy informal care costs associated with institutionalized people with AD-Dementia were also not available from the literature. These values were calculated using a foregone wage approach under the assumption that care partners would visit relatives 3 hours weekly, over 52 weeks. Each hour was valued using country-specific average wages (48). Selected cost inputs, disaggregated by AD severity and type of cost are shown in Table 4 of the Supplemental Materials.
Results
Model Outputs
In this study, we have estimated the present value of the lifetime AD-related costs and life years for a cohort of individuals aged 65 years and over with prevalent MCI-AD across six European countries. Below we present aggregate ‘pan-European’ data for these six countries to estimate a pan-European model. Country-level data can be found in the Supplementary Materials along with data on the US and Canada for comparison.
Published age-specific MCI-AD prevalence rates were used to calculate the size of the cohort with MCI-AD. We then used a published Markov model structure to replicate the natural history of AD to model the progression of this cohort through the AD continuum over time (see Methods). Total costs and life years were then calculated for the lifetime of the cohort and discounted at a rate of 3% to produce a present value of
both costs and life years for a cohort aged 65 years and over.
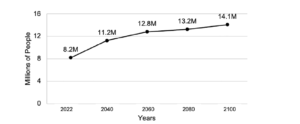
We estimate that the pan-European prevalence of MCI-AD in 2022 is 8.2M (see Figure 1). Figure 2 illustrates the present value of costs for this 2022 cohort, broken down by disease stage and type of cost (i.e. direct medical, direct non-medical, informal). In the absence of any intervention, the present (2022) value of total lifetime costs for this cohort moving through all disease stages is ~€1.2T. Our model predicts that approximately 43% (€507B) of the total present value of these costs is driven by patients with MCI-AD with the remainder due to patients in AD-Dementia stages (€665.6B). Importantly, echoing the relatively small contribution of direct medical costs in AD (43), approximately 80% of the present value of lifetime costs in our model are driven by informal care and non-medical direct costs (see Figure 2).
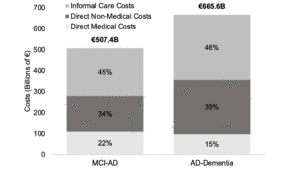
Figure 2
Present value of the lifetime AD-related costs by cost type for a cohort of patients aged 65 and over with prevalent MCI-AD in 2022
To explore the impact of changing demographics on future AD burden, we used published population projections to estimate the future prevalence of MCI-AD and repeated the above-described analysis for every year up to 2100. In the absence of any interventions, the prevalence of MCI-AD is expected to increase by 72% by 2100, reaching approximately 14.1M people (see Figure 1). Figure 3 depicts the present (2022) value of lifetime costs and life years for a cohort aged 65 years and over starting at selected time points up until the year 2100, for both MCI-AD and AD-Dementia stages. The latter aggregates mild, moderate, and severe AD stages for presentation purposes. Notably, driven by the increased prevalence of MCI-AD, the total pan-European present value of AD-related costs is expected to increase by approximately 40% to €1.6T in 2100.
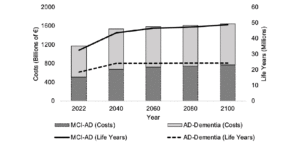
Figure 3
Present value of the lifetime AD-related costs and life years for a cohort of patients aged 65 years and over with
prevalent MCI-AD up to 2100
Exploring the Impact of Early Intervention
Our model allows for the assessment of the potential impact of early intervention with a hypothetical new DMT or potentially preventative lifestyle-based modifications.
To assess the economic consequences of a hypothetical DMT, we explored the impact of slowing disease progression between MCI and all AD-Dementia stages by an assumed 25% and 50%. Table 1 present the impact of these two scenarios on the present value of costs and life years for MCI-AD and AD-Dementia stages at select time points up to the year 2100. Figure 4 visually illustrates the impact of these two scenarios on the present value of costs associated with the 2022 cohort described above. Reducing disease progression rates increases the relative life years, and hence costs, associated with MCI-AD, in which patients are relatively more independent and have a higher quality of life compared to the more severe stages of the disease, while simultaneously decreasing the number of life years and associated costs of AD-Dementia stages. Nonetheless, our model suggests that the increase in costs associated with MCI-AD is offset by savings due to a reduction of time and costs spent in the AD-Dementia stages, which translates into overall cost savings of €33.7B and €72.7B for 2022 for the 25% and 50% reduction in progression scenarios (see Table 1); in percentage terms, this translates to costs savings of ~3% and ~6% for 2022, respectively; however, our analysis does not factor in the acquisition costs of a DMT.
We also conducted a separate scenario to understand the economic consequences of halting MCI-AD progression for 3 years (with no therapeutic effect thereafter) through a potential lifestyle-based intervention program. The outputs of this scenario are comparable to the 50% reduction in disease progression described above (see Table 1 and Figure 4). Overall cost savings of €84.7B in savings in 2022 are predicted in this scenario, which translates to cost savings of ~7%.
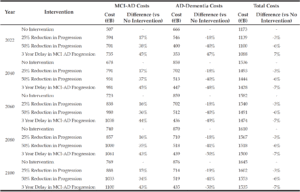
Table 1
Three scenarios assessing the impact of reducing disease progression and delaying disease onset on the present value of the lifetime AD-related costs annually up to the year 2100
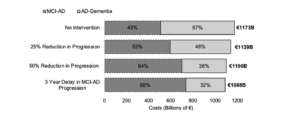
Figure 4
Three scenarios reducing disease progression and delaying disease onset on the present value of the lifetime
AD-related costs for a cohort aged 65 years and over with prevalent MCI-AD in 2022
Discussion
This study estimates the lifetime value of AD-related costs for a cohort of patients aged 65 years or older receiving a diagnosis of MCI-AD in 2022. We project these modelled outcomes into the future accounting for expected demographic changes and also explore the potential economic impact of hypothetical interventions. Below we discuss the implications of our results, review our approach and its associated strengths and limitations, and close with recommendations for future research.
The Burden of AD
Our results predict that the expected present value of the lifetime AD-related costs for a cohort aged 65 years and older with prevalent MCI-AD in 2022 is €1.2T across the six European countries examined—a figure comparable to the gross domestic product of Spain (51). In line with previous studies, direct medical costs to the healthcare system only represent ~20% of total costs, with social and informal care sectors instead shouldering most of the burden. Moreover, our results suggest that a significant component of the total costs of AD is due to MCI-AD (43% of total costs), primarily due to life years in the MCI-AD stage being close to double the life years in the AD-Dementia stage (see Figure 3). As noted above, estimating the precise prevalence and costs associated with MCI-AD is challenging, yet several studies have found that the costs are elevated in MCI-AD stages and that costs can begin to rise many years prior to an official diagnosis of AD (43, 52, 53). For example, using records from the Danish National Patient Registry, increased healthcare costs were identified in patients as early as 10 years prior to a diagnosis of AD (54). As recently argued, the observation of increased costs in MCI-AD or preclinical stages is in line with the thinking that AD is a pathological continuum and that such costs may be substantial and ‘hidden’ from economic estimates of the burden of AD (43).
The Impact of Early Interventions, Including Potential Lifestyle-Based Modifications and DMTs
Several studies have identified modifiable risk factors for AD, such as physical inactivity, smoking, and hypertension, raising the intriguing possibility of preventing or delaying AD cognitive decline and dementia through behavioral or lifestyle-based changes; for example, the Lancet Commission recently suggested that up 40% of global dementia cases could potentially be delayed or prevented by interventions targeted at identified 12 modifiable risk factors (7). Moreover, multidomain lifestyle-based modification trials have shown that such interventions can result in cognitive benefits in individuals with a higher risk of dementia (8). In line with this emerging body of evidence, the WHO has released guidelines on risk reduction of cognitive decline and dementia and provides evidence-based recommendations on lifestyle-based behaviours and interventions (57).
There has been growing interest in understanding the potential economic impact of such interventions (15). For example, one study found that savings of US$110 billion could potentially be realized from a 10% reduction in four risk factors (high body-mass index, diabetes, hypertension, and cardiovascular diseases). Similarly, Mukadam et al. (2020) recently found that preventative interventions (targeting hypertension, reducing smoking, providing hearing aids) could save almost £2 billion annually in England and reduce dementia prevalence by 8.5%. Our results are in line with these findings (15).
Our data further suggest that early intervention with a hypothetical DMT that either reduces or delays progression from MCI-AD stages to AD-Dementia could result in significant cost savings for society. This finding is in line with those of Cimler et al. (2019), who demonstrated that prolonging the stay of ‘not-yet patients’ in the MCI-AD stage could result in substantial cost savings (14). Collectively, our findings provide support for a growing body of literature highlighting the importance of early detection and intervention in AD via potential DMTs or lifestyle-based behavioral modifications (58, 59).
Interestingly, our modelling approach, which focused on economic costs, may underestimate the burden on society and potential value that an effective intervention could bring. For example, recent studies have explored what actually matters to patients and their care partners and what could constitute a meaningful delay in disease progression from their perspective (1, 55, 56). These studies have found that ‘humanistic’ impacts of the disease in areas such as independence, emotions, mood and social lives, which deteriorate in advanced disease stages, are important considerations for patients and their loved ones (44, 55). This suggests that a treatment that prolongs an individual’s stay in earlier, less severe disease stages could bring significant benefits beyond monetary cost savings.
Strengths and Limitations of Our Approach
As outlined above, we started with a cohort with prevalent MCI-AD in an attempt to model all transitions according to the natural history of the disease. This approach, therefore, estimates the population-level lifetime costs across the AD continuum. Moreover, by combining demographic projections and AD progression modelling, our study incorporates the consequences of changing demographics in several European countries. From a policy perspective, we believe that this is an informative analysis as it represents the total costs of AD associated with the entire cohort of people across the entire disease continuum.
We also include the costs of MCI-AD in our analysis in contrast to existing reports that focus primarily on AD-Dementia (11, 13, 60). We believe that including MCI-AD is important given the emerging evidence that elevated costs associated with AD can occur several years prior to diagnosis (discussed further below) and due to the change in the medical paradigm emphasizing diagnosis and treatment at the preclinical and prodromal stages of AD (61). However, uncertainty exists around the true estimates of the prevalence of MCI-AD, which is a limitation of this and other epidemiological models. Another weakness of our analysis is the number of assumptions required to estimate cost inputs for people at the MCI-AD stage and costs associated with informal care in the institutional setting (see Methods). This results from major gaps in the available literature which will hopefully be addressed by future research. Nonetheless, the current approach accounts for the effects of age on the natural history of AD by utilizing age-specific inputs to calculate the current and projected prevalence of AD in the entire country’s population and to predict AD-severity progression. It also allows modeling mortality more naturally, as the excess AD mortality is applied to general population age-death rates. In our view, these are the strengths of the analysis contributing to greater precision in our estimates.
Comparisons of the absolute results of our analysis to those from other studies should consequently be made with caution given the different methodologies used. Firstly, unlike other published reports, we have included estimates of the costs associated with MCI-AD. Secondly, we model AD progression departing from the MCI-AD stage diagnosed at the ages of 65 and above, and report lifetime costs for the cohort. Deriving an annual average cost using our results would depend on the selected denominator (life years lived, analysis time horizon) and would probably differ from annual cross-sectional estimates produced by models assuming a fixed onset of disease. Ultimately, the effect of age-specific and excess AD-related mortality on our cohort’s survival produces age and severity adjusted costs of AD that will differ from other modeling strategies. The decision lies on researchers and policymakers to reflect on the various modelling tools available and leverage the methodology most suited to their needs.
Recommendations for Future Research
The societal burden of AD is growing and has the potential to overwhelm systems of care (43). Moreover, several recent publications have brought a critical lens to the way the burden of AD is measured, and have highlighted the unique challenges and limitations of health economic modelling for this disease and evaluating the impact of interventions (44, 62, 63). Accurate estimates of the burden of AD are critical to guiding policy and investment decisions. We reiterate that there is a need for better data on disease prevalence and the range and type of costs across the entire AD continuum, particularly in the MCI-AD and preclinical phases. Recent and ongoing work through the ROADMAP initiative may help close some of these critical knowledge gaps (56, 64). Echoing previous reports, our findings further reinforce the importance of accounting for the full value of innovative treatments, management and care paradigms, including their potential impact on direct, indirect and intangible costs affecting patients and their families (43). We believe that this study and our modelling approach add to the growing toolkit that stakeholders can rely on to estimate the societal burden of AD and evaluate the potential impact of novel interventions, including potential DMTs and lifestyle-based interventions.
Conflict of interest: Rui Martins reports grants from Biogen International GmbH during the conduct of the study; and grants from Biogen International GmbH outside the submitted work. Michael Urbich is an employee of Biogen International GmbH. At the time of writing the manuscript, Karin Brännvall was an employee and stockholder of Biogen International GmbH. Mattia Gianinazzi is an employee and stockholder of Biogen International GmbH. Jamie Ching, Charles Khoury and Youssef El-Hayek report funding from Biogen International GmbH during the conduct of the study; other funding from Biogen International GmbH and from F. Hoffman-La Roche Ltd outside the submitted work; and are employees of Shift Health, providing consultancy services to organizations across the health and life sciences sector. All authors contributed to the design of this study, the collection, analysis, and interpretation of data, and the preparation and review of the manuscript.
Acknowledgments: The authors would like to acknowledge William L. Herring, PhD for developing the Markov traces used in this study.
Funding sources: This study was financially sponsored by Biogen International GmbH.
References
1. Jessen F, Georges J, Wortmann M, Benham-Hermetz S. What Matters to Patients with Alzheimer’s Disease and Their Care Partners? Implications for Understanding the Value of Future Interventions. J Prev Alzheimer’s Dis 2022;1-6.
2. Bowling A, Rowe G, Adams S, et al. Quality of life in dementia: A systematically conducted narrative review of dementia-specific measurement scales. Aging Ment Heal 2015;19:13-31.
3. Jones RW, Romeo R, Trigg R, et al. Dependence in Alzheimer’s disease and service use costs, quality of life, and caregiver burden: The DADE study. Alzheimer’s Dement 2015;11:280-90.
4. WHO reveals leading causes of death and disability worldwide: 2000-2019. https://www.who.int/news/item/09-12-2020-who-reveals-leading-causes-of-death-and-disability-worldwide-2000-2019. Accessed 4 April 2022.
5. Yiannopoulou KG, Papageorgiou SG. Current and Future Treatments in Alzheimer Disease: An Update. J Cent Nerv Syst Dis 2020.
6. Liss JL, Seleri Assunção S, Cummings J, et al. Practical recommendations for timely, accurate diagnosis of symptomatic Alzheimer’s disease (MCI and dementia) in primary care: a review and synthesis. J Intern Med 2021;1-25.
7. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020;396(10248):413-46.
8. Scheltens P, De Strooper B, Kivipelto M, et al. Alzheimer’s disease. Lancet 2021;397(10284):1577-90.
9. Alzheimer’s Association. 2020 Alzheimer’s disease facts and figures. Alzheimer’s Dement 2020;16:391-460.
10. Cummings J, Lee G, Zhong K, Fonseca J, Taghva K. Alzheimer’s disease drug development pipeline: 2021. Alzheimer’s Dement Transl Res Clin Interv 2021;7.
11. Alzheimer’s Association. Changing the Trajectory of Alzheimer’s Disease: How a Treatment by 2025 Saves Lives and Dollars. 2015. https://www.alz.org/media/documents/changing-the-trajectory-r.pdf. Accessed 4 February 2022.
12. Prince M, Wimo A, Guerchet M, Ali GC, Wu YT, Prina M. World Alzheimer Report 2015. 2015. https://www.alzint.org/u/WorldAlzheimerReport2015.pdf. Accessed 6 October 2021.
13. Wimo A, Guerchet M, Ali GC, et al. The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimer’s Dement 2017;13:1-7.
14. Cimler R, Maresova P, Kuhnova J, Kuca K. Predictions of Alzheimer’s disease treatment and care costs in European countries. PLoS One 2019;14:e0210958.
15. Mukadam N, Anderson R, Knapp M, et al. Effective interventions for potentially modifiable risk factors for late-onset dementia: a costs and cost-effectiveness modelling study. Lancet Heal Longev 2020;1(1):e13-20.
16. Herring WL, Gould IG, Fillit H, et al. Predicted Lifetime Health Outcomes for Aducanumab in Patients with Early Alzheimer’s Disease. Neurol Ther 2021;10:919-940.
17. EuroStat. EUROPOP2019 – Population projections at national level (2019-2100). 2021. https://ec.europa.eu/eurostat/databrowser/view/PROJ_19NP__custom_1799875/default/table?lang=en. Accessed 12 December 2021.
18. Office for National Statistics. National Population projections by single year of age. 2021. https://www.nomisweb.co.uk/query/construct/summary.asp?mode=construct&version=0&dataset=2009. Accessed 12 December 2021.
19. Petersen RC, Lopez O, Armstrong MJ, et al. Practice guideline update summary: Mild cognitive impairment: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 2018;90:126-35.
20. Knopman DS, Gottesman RF, Sharrett AR, et al. Mild Cognitive Impairment and Dementia Prevalence: The Atherosclerosis Risk in Communities Neurocognitive Study (ARIC-NCS). Alzheimers Dement (Amst) 2016;2:1-11.
21. Haacker M, Hallett TB, Atun R. On discount rates for economic evaluations in global health. Health Policy Plan 2020;35:107-14.
22. DESTATIS. Consumer price index: Germany, months, individual consumption by purpose (COICOP 2-5-digit hierarchy). 2021. https://www-genesis.destatis.de/genesis/online?sequenz=tabelleErgebnis&selectionname=61111-0004&sachmerkmal=CC13A5&sachschluessel=CC13-06*&zeitscheiben=3&language=en. Accessed 25 January 2022.
23. INSEE. Annual HICP – Reference year 2015=100 – All households – France – COICOP classification: 06 – Health – Identifier 001763105. 2021. https://www.insee.fr/en/statistiques/serie/001763105. Accessed 25 January 2022.
24. European Central Bank. Italy – HICP – HEALTH, Monthly Index, Eurostat, Neither seasonally nor working day adjusted. 2021. https://sdw.ecb.europa.eu/quickview.do?SERIES_KEY=122.ICP.M.IT. N.060000.4.INX&start=1996&end=&submitOptions.x=0&submitOptions.y=0&trans=AF. Accessed 25 January 2022.
25. Instituto Nacional de Estadística. Consumer price index. Base 2016. Annual averages. 2021. https://www.ine.es/jaxiT3/Datos.htm?t=22553. Accessed 25 January 2022.
26. Statistics Sweden. CPI, Indices for Main Groups, annual averages. 2021. https://www.scb.se/en/finding-statistics/statistics-by-subject-area/prices-and-consumption/consumer-price-index/consumer-price-index-cpi/pong/tables-and-graphs/consumer-price-index-cpi/cpi-indices-for-main-groups-annual-averages/. Accessed 25 January 2022.
27. Curtis LA, Burns A. Unit Costs of Health and Social Care 2017. Kent Acad Repos. 2017. https://kar.kent.ac.uk/65559/. Accessed 4 February 2022.
28. Jones KC, Burns A. Unit Costs of Health and Social Care 2021. Personal Social Services Research Unit. 2021. https://kar.kent.ac.uk/92342/1/Unit Costs Report 2021 – Final version for publication.pdf. Accessed 4 February 2022.
29. Bank of America. Currency Calculator: Rates for Ordering Foreign Currency (US dollars to Euros). 2021. https://www.bankofamerica.com/foreign-exchange/currency-converter/. Accessed 13 December 2021.
30. Bank of England. Daily spot exchange rates against Sterling (British Pounds to Euros). 2021. https://www.bankofengland.co.uk/boeapps/database/Rates.asp?Travel=NIxAZx&into=GBP. Accessed 13 December 2021.
31. Green C, Handels R, Gustavsson A, et al. Assessing cost-effectiveness of early intervention in Alzheimer’s disease: An open-source modeling framework. Alzheimers Dement 2019;15:1309-21.
32. Wimo A, Handels R, Winblad B, et al. Quantifying and Describing the Natural History and Costs of Alzheimer’s Disease and Effects of Hypothetical Interventions. J Alzheimers Dis 2020;75:891-902.
33. Davis M, Thomas OC, Johnson S, et al. Estimating Alzheimer’s Disease Progression Rates from Normal Cognition Through Mild Cognitive Impairment and Stages of Dementia. Curr Alzheimer Res 2018;15:777-88.
34. Santabárbara J, Gracia-García P, Pírez G, et al. Mortality in mild cognitive impairment diagnosed with DSM-5 criteria and with petersen’s criteria: A 17-year follow-up in a community study. Am J Geriatr Psychiatry 2016;24:977-86.
35. Andersen K, Lolk A, Martinussen T, Kragh-Sørensen P. Very mild to severe dementia and mortality: A 14-year follow-up – The Odense study. Dement Geriatr Cogn Disord 2010;29:61-7.
36. DESTATIS. Life table (period life table): Germany, period of years, sex, completed age 2018-2020. 2021. https://www-genesis.destatis.de/genesis/online?sequenz=statistikTabellen&selecctionname=12621&language= en#abreadcrumb. Accessed 25 January 2022
37. I.Stat. Life tables 2020. 2021. https://www.google.com/ search?q=Istat&oq=Istat&aqs=chrome.69i57j35i39j69i60l4j69i6 1l2.1088j0j7& sourceid=chrome&ie=UTF-8.. Accessed 27 October 2021.
38. Instituto Nacional de Estadística. Population mortality tables for Spain by year, sex, age and functions. 2021. https://www.ine.es/jaxiT3/Tabla.htm?t=27153. Accessed 27 October 2021.
39. Institut National d’Études Démographiques. Mortality tables 2017-2019. 2021. https://www.ined.fr/fichier/s_rubrique/193/fm_t68_2019.en.xlsx. Accessed 27 October 2021.
40. Office for National Statistics. National life tables: UK 2018-2020. 2021. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/datasets/nationallifetablesunite dkingdomreferencetables. Accessed 27 October 2021.
41. Statistics Sweden. Lifespan in Sweden 2011–2020 Life expectancy tables for the country and the counties. 2021. https://www.scb.se/contentassets/f71836afa6a54689973eb6336347650c/be0701_2011i20_br_be51br2104.pdf. Accessed 21 October 21 2021.
42. Cantarero-Prieto D, Leon PL, Blazquez-Fernandez C, Juan PS, Cobo CS. The economic cost of dementia: A systematic review. Dementia 2020;19:2637-57.
43. El-Hayek YH, Wiley RE, Khoury CP, et al. Tip of the Iceberg: Assessing the Global Socioeconomic Costs of Alzheimer’s Disease and Related Dementias and Strategic Implications for Stakeholders. Journal of Alzheimer’s Disease 2019;70:323-341.
44. Kließ MK, Martins R, Connolly MP. Major Cost Drivers in Assessing the Economic Burden of Alzheimer’s Disease: A Structured, Rapid Review. J Prev Alzheimer’s Dis 2021;8:362-70.
45. Darbà J, Kaskens L, Lacey L. Relationship between global severity of patients with Alzheimer’s disease and costs of care in Spain; results from the co-dependence study in Spain. Eur J Heal Econ 2015;16:895-905.
46. Olazarán J, Agüera-Ortiz L, Argimón JM, et al. Costs and quality of life in community-dwelling patients with Alzheimer’s disease in Spain: Results from the GERAS II observational study. Int Psychogeriatrics 2017;29:2081-93.
47. Wübker A, Zwakhalen SMG, Challis D, et al. Costs of care for people with dementia just before and after nursing home placement: primary data from eight European countries. Eur J Heal Econ 2015;16(7):689-707.
48. OECD. Average annual wages. 2021. https://data.oecd.org/earnwage/average-wages.htm. Accessed 14 December 2021.
49. Robinson RL, Rentz DM, Andrews JS, et al. Costs of Early Stage Alzheimer’s Disease in the United States: Cross-Sectional Analysis of a Prospective Cohort Study (GERAS-US)1. J Alzheimers Dis 2020;75:437-50.
50. Prince M, Knapp M, Guerchet M, et al. Dementia UK Update. 2014; https://www.alzheimers.org.uk/sites/default/files/migrate/downloads/dementia_uk_update.pdf. Accessed 4 February 2022.
51. World Bank. GDP (current US$) | Data. https://data.worldbank.org/indicator/NY.GDP.MKTP.CD. Accessed 15 February 2022.
52. Lin PJ, Zhong Y, Fillit HM, Chen E, Neumann PJ. Medicare Expenditures of Individuals with Alzheimer’s Disease and Related Dementias or Mild Cognitive Impairment Before and After Diagnosis. J Am Geriatr Soc 2016;64:1549-57.
53. Zhu CW, Sano M, Ferris SH, Whitehouse PJ, Patterson MB, Aisen PS. Health-Related Resource Use and Costs in Elderly Adults with and without Mild Cognitive Impairment. J Am Geriatr Soc. 2013;61:396.
54. Frahm-Falkenberg S, Ibsen R, Kjellbrg J, Jennum P. Health, social and economic consequences of dementias: a comparative national cohort study. Eur J Neurol 2016;23:1400-7.
55. Dibenedetti DB, Slota C, Wronski SL, et al. Assessing what matters most to patients with or at risk for Alzheimer’s and care partners: A qualitative study evaluating symptoms, impacts, and outcomes. Alzheimer’s Res Ther 2020;12:1-15.
56. Tochel C, Smith M, Baldwin H, et al. What outcomes are important to patients with mild cognitive impairment or Alzheimer’s disease, their caregivers, and health-care professionals? A systematic review. Alzheimer’s Dement Diagnosis, Assess Dis Monit 2019;11:231-47.
57. Risk reduction of cognitive decline and dementia: WHO guidelines. https://www.who.int/publications/i/item/9789241550543. Accessed 14 September 2022.
58. Aisen PS, Cummings J, Jack CR, et al. On the path to 2025: Understanding the Alzheimer’s disease continuum. Alzheimer’s Res Ther. 2017;9:1-10.
59. Bronner K, Perneczky R, McCabe R, Kurz A, Hamann J. Which medical and social decision topics are important after early diagnosis of Alzheimer’s Disease from the perspectives of people with Alzheimer’s Disease, spouses and professionals? BMC Res Notes 2016;9:1-10.
60. Zetterberg H, Burnham SC. Blood-based molecular biomarkers for Alzheimer’s disease. Mol Brain 2019;12:26.
61. Dubois B, Hampel H, Feldman HH, et al. Preclinical Alzheimer’s disease: Definition, natural history, and diagnostic criteria. Alzheimer’s Dement 2016;12:292-323.
62. Gustavsson A, Green C, Jones RW, et al. Current issues and future research priorities for health economic modelling across the full continuum of Alzheimer’s disease. Alzheimer’s Dement 2017;13:312-21.
63. Gustavsson A, Pemberton-Ross P, Gomez Montero M, Hashim M, Thompson R. Challenges in demonstrating the value of disease-modifying therapies for Alzheimer’s disease. Expert Rev Pharmacoeconomics Outcomes Res2020;20:563-70.
64. Janssen O, Vos SJB, García-Negredo G, et al. Real-world evidence in Alzheimer’s disease: The ROADMAP Data Cube. Alzheimer’s Dement 2020;16:461-71.