C. Demczar1, C. Behrens2, L. Baltz2, V.P. Georgescu1, D. Arminavage1, J.M. Brown1, D. Fussell1, T.C. Trate3, S.R. McAnulty1, L.S. McAnulty2, E.K. Merritt1
1. Department of Health and Exercise Science, Beaver College of Health Sciences, Appalachian State University; 2. Department of Nutrition and Health Care Management, Beaver College of Health Sciences, Appalachian State University; 3. Appalachian Rehabilitation, Boone, NC
Corresponding Author: Edward K. Merritt, ASU Box 32071, 111 Rivers St., Boone, NC 28608, Phone: (828) 262-7986, Fax: (828) 262-3138, merritte@southwestern.edu
J Aging Res Clin Practice 2017;6:229-237
Published online November 23, 2017, http://dx.doi.org/10.14283/jarcp.2017.31
Abstract
Muscles of old adults respond to stress with heightened inflammatory signaling that disrupts the regenerative process. This muscle inflammation susceptibility could contribute to the age-related decline in muscle mass, as anti-inflammatory medications taken concurrently with exercise training, have proven beneficial in attenuating age-related loss of muscle mass. With antioxidants and anti-inflammatory potential, blueberries (BB) are a natural alternative that might regulate aged muscle inflammation susceptibility. Objectives: The purpose of this study was to determine the effects of BB consumption on the muscle inflammatory profile of older adults, and to determine the subsequent muscle inflammatory response to exercise. We hypothesized that BB would lower the inflammatory profile of muscle and attenuate the inflammatory response after resistance exercise. Design: Subjects were randomized to receive daily BB or placebo supplements, which were blind to subjects and researchers. All subjects underwent baseline functional testing, post-supplementation testing, and testing post-muscle stress stimulus. Setting: Volunteers were recruited from Western North Carolina region, USA. Participants: Healthy, non-resistance trained adults over 60 years old (n=22) were recruited. Measurements: Profiles of inflammation pathways known to affect muscle mass were established prior to and after 6-weeks of daily consumption of BB. Post-supplementation, subjects performed an exercise protocol to induce inflammation and returned 24 hours post-exercise to determine the muscle inflammatory profiles. Results: Muscle cytokine and soluble cytokine receptor levels were similar between groups and within groups before and after BB consumption. Cytokine and cytokine receptor levels post-muscle stress changed similarly in the BB and placebo group, indicating BB had no effect on the muscle’s inflammatory response. Total plasma antioxidant capacity was 22% higher in the BB group 24-hours post-muscle stress, however, plasma oxidative stress was not different between groups or within groups. Conclusion: While BB consumption did not affect inflammatory signaling pathways within the muscle nor affect inflammation after a regenerative stimulus, a higher plasma antioxidant capacity could contribute to a better long-term regenerative response.
Key words: Skeletal muscle, inflammation susceptibility, blueberries, aging, anti-inflammatory.
Introduction
The age-related loss of skeletal muscle mass, termed sarcopenia, occurs gradually over time and is strongly associated with a progressive decline in physical capacity and can lead to disability and loss of functional independence. The etiology of aging atrophy is poorly understood. Among the potential causes that continue to be explored, an area of interest involves muscle regenerative biology. Aging atrophy might in part result from episodes of incomplete muscle repair throughout adulthood. Whether this is a major cause of atrophy of the normal aging muscle is debatable, but there is no question that skeletal muscle regenerative capacity declines with age as is consistently shown following overt muscle damage (1, 2).
Based on research findings to date, it is theorized that muscles of old (vs. young) suffer heightened and prolonged pro-inflammatory and proteolytic signaling that disrupts the local environment leading to failed myogenesis, and ultimately, a decline in muscle mass for a given concentration of cytokines in the circulation or local interstitial compartment. Evidence has been obtained in vitro and in vivo, to further suggest that this is the case. Skeletal muscle from healthy, older humans has an elevated local inflammatory profile when compared to young, despite the lack of evidence of systemic inflammation (3, 4). Bolstering this theory is the evidence that pharmacological inhibitors of the cyclooxygenase inflammatory pathway enhance gains in muscle size and strength in older individuals undergoing resistance training, but not young adults (5). Additionally, in vitro, muscle progenitor cells from older adults respond with a heightened inflammation response compared to younger adults when subjected to an identical concentration of inflammatory cytokines. These findings have broad implications for aged skeletal muscle research and might help explain the cause of sarcopenia as well as the blunted regenerative response to injury that has been documented in older adults (4).
Preventing local muscle inflammation is an obvious way to further test the aged muscle inflammation susceptibility theory. Blueberries, with high concentrations of antioxidants and anti-inflammatory compounds such as anthocyanins, are an ideal natural, non-pharmacologic candidate to test the theory. Evidence exists that other fruits with anti-inflammatory compounds are beneficial for muscle recovery following damage in young adults [6]. However, it is unknown how the muscle’s inflammatory profile and cellular response to damage were affected, nor is it known if the fruit would benefit older adults in a similar manner. Therefore, the purpose of this study was two-fold: 1) To determine the effects of 6 weeks of blueberry supplementation on the systemic and local muscle inflammatory profile of older adults; and 2) To measure the inflammatory/regenerative response of the muscle following a regenerative stimulus with or without blueberry supplementation. We hypothesized that 6-weeks of blueberry supplementation would lower the basal inflammatory profile of aged skeletal muscle, and would subsequently attenuate the heightened inflammatory response normally observed after a stressful bout of resistance exercise.
Materials and Methods
Subjects
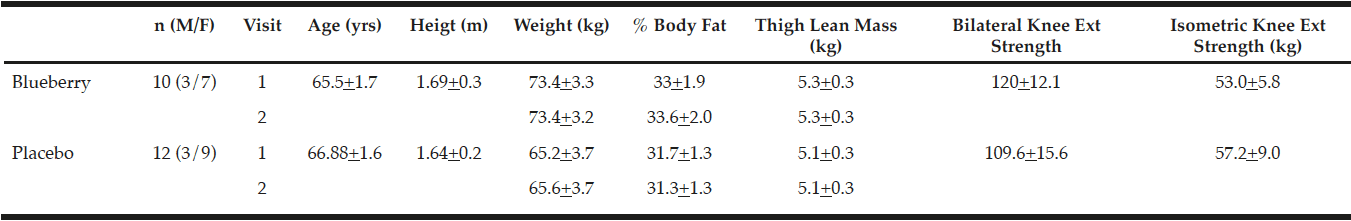
Twenty-four men and women over 60 years old were recruited to partake in this study (Table 1). Volunteers were screened with a health history questionnaire and allowed to participate if they were apparently healthy and did not participate in a regular exercise program that included any form of resistance exercise or high impact aerobic activity such as running for six months prior to the study and for the duration of the study Subjects were excluded from the study if they had a body mass index greater than 30, used any type of anti-inflammatory medications or suffered from a disease characterized by a chronic inflammatory state, had uncontrolled, severe hypertension, a lidocaine allergy, or were told by their doctor that they should not engage in strenuous exercise. The subjects further agreed not to alter any lifestyle habits during the study, as well as refrain from daily use of non-steroidal anti-inflammatory drugs or other medications that may impact any aspect of the study. All subjects signed a written informed consent document approved by the local Institutional Review Board, and were informed of all protocols, procedures, and potential risks associated with the study.
Study Design
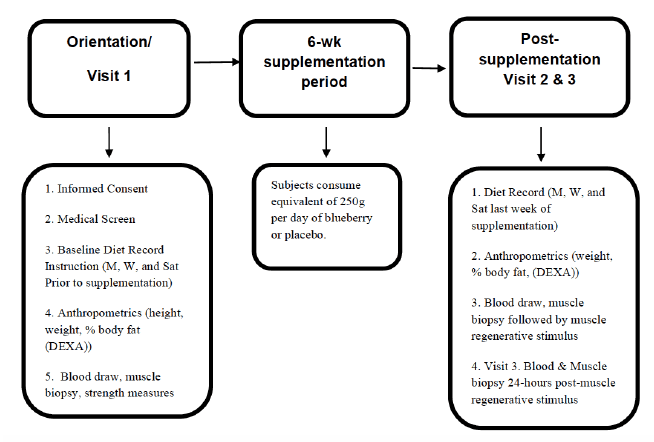
This study consisted of three visits, the second occurred six weeks after the first visit, and the third occurred 24 hours following the second visit (See Figure 1). During visit 1, informed consent and the medical screen were completed, followed by baseline measurements. These included height, weight, body composition, a blood draw, muscle biopsy, and strength measurements (described below). In addition, diet recording instructions were explained to the subject, where the first diet baseline was taken three days prior to starting supplementation. Subjects were randomized to the blueberry or placebo group. Between visits 1 and 2, the subjects consumed the supplement or placebo. Following the 6 week supplementation period, the subjects returned for a visit 2. During visit 2, anthropometric measures and diet records from the previous week were collected. A blood draw and muscle biopsy were then performed. The subject then underwent a mechanically-induced muscle stress protocol as a stimulus for inflammation and subsequent regeneration. Subjects returned to the laboratory for visit 3, 24 hours after visit 2. The final blood and muscle biopsy samples were obtained at this time.
Body Composition Assessment
Dual energy X-ray absorptiometry (DXA: Discovery W (S/N 81225); Hologic, Inc. Marlborough, MA) was performed to determine whole body fat and lean mass, thigh muscle mass, and body fat percentage according to manufacturer’s instructions.
Strength Measurements
Two strength measurements were taken during visit 1. A maximal isometric knee extension utilizing the right leg of each subject was taken using a transistor that was amplified by and converted by a DAQ board (National Instruments, Austin, TX) to give a quantitative output in Newtons (LabView, National Instruments, TX). Up to five trials were recorded, with the maximum value obtained being considered as the subjects isometric knee extension maximum force. The second strength measurement was a one repetition maximal effort knee extension exercise completed on a Cybex Isotonic Knee Extension machine. Initial resistance was estimated using the isometric strength value converted to pounds, and weight was added until a single repetition could not be completed with proper form. This value was considered the subject’s knee extension one repetition maximum and was used to determine the resistance used for the muscle stress protocol (60% of maximum).
Diet Education/Analysis
Each subject was informed on how and when to accurately record dietary intake on a three-day diet record during the first and final week of supplementation. On visit 2, the returned diet records were reviewed with the subject to ensure completeness and clarity. Data from diet records were entered into diet analysis software (The Food Processor SQL – Version 10.12.0, ESHA Research, Salem, Oregon). Detailed printouts of nutrient intake were compared to written entries by another member of the research team to identify errors in entry and ensure accurate data entry. Data from three-day diet records were analyzed to determine differences in dietary intake within and between groups at baseline and 6-weeks post-supplementation.
Blueberry/Placebo Supplementation
The supplementation protocol consisted of subjects ingesting 100% freeze-dried blueberry powder [U.S. High Bush Blueberry Council (USHBC), Folsom, CA, 50/50 blend of Tifblue (Vaccinium virgatum [ashei]) and Rubel (Vaccinium corymbosum)] or placebo (maltodextrin, fructose, artificial flavoring, artificial purple and red color, citric acid, and silica dioxide) daily for six weeks. Subjects consumed 38 g of powder, equivalent to approximately 250 g of whole blueberries. Specific nutritional data for the powders was previously reported (7). Blueberry and placebo packets coded by the USHBC to blind researchers and subjects to their assignment were apportioned into labeled week-by-week bags. Subjects were instructed to consume packets with 8-ounces of water with their evening meal, but were told to avoid consuming dairy products with the supplement. Compliance was confirmed via weekly e-mail/phone correspondence, and subjects were instructed to return supplement packaging, empty or otherwise, to its respective weekly bag for return at the conclusion of the study. Upon return, packets were counted to determine compliance.
Blood and Muscle Sampling
Blood sampling occurred in the morning when the subjects were in a fasted, rested state. Approximately 10-ml of blood was collected from an antecubital vein into heparinized and EDTA vacutainer tubes. The tubes were immediately placed on ice and then spun at 1000g for 10 min at 4 °C. The plasma from the heparin tubes was aliquoted into cryotubes, frozen in liquid nitrogen, and stored at –80 °C until analyses.
Muscle biopsies were taken immediately following the blood draws. A total of three biopsies were obtained, one during each visit; visit 1, before supplementation, visit 2, after the 6-weeks of supplementation, and visit 3, 24-hours post muscle stress exercise. Muscle samples were taken from the m. vastus lateralis under local anesthetic (1% lidocaine) by percutaneous needle biopsy. The contralateral limb was used for the post-muscle damage biopsy. Samples were snap frozen in liquid nitrogen and stored at -80°C until analysis.
Mechanically-Induced Muscle Damage
Subjects performed 9 sets of ten repetitions of a bilateral knee extension exercise using a resistance equivalent to 60% of that subject’s one repetition maximum (1RM), as determined by the isotonic knee extension strength measurement. Similar protocols were found sufficient to induce an inflammatory response and moderate damage to myofibers in untrained subjects (4). Subjects were instructed to perform the isotonic knee extension emphasizing a fast concentric phase and a slow eccentric phase at approximately a 1:4 time ratio. A one minute rest period was given between sets. If the subject was unable to finish all ten repetitions of the previous set a longer rest was allowed rather than lowering the weight.
Plasma oxidative stress measurement
F2-isoprostanes were determined using an enzyme linked immunosorbent assay (ELISA) kit following the manufacturer’s instructions (Cayman Chemical #516360, Ann Arbor, MI). Briefly, this is a competitive assay with a range of 2.5 to 1,500 pg/mL and a sensitivity (80% B/Bo) of about 10 pg/mL.
Plasma antioxidant potential
Total plasma antioxidant potential was determined by the ferric reducing ability of plasma (FRAP) assay according to the methodology of Benzie et al. (8). The basis of this assay is that water soluble reducing agents (antioxidants) in the plasma will reduce ferric ions to ferrous ions, which then react with an added chromogen. Samples and standards were analyzed in duplicate and expressed as ascorbate equivalents based on an ascorbate standard curve (0-1000 µmol/L). Intra-assay and inter-assay coefficients of variation were less than 5% and 7%, respectively.
Plasma creatine kinase activity
Plasma creatine kinase activity was measured on visit 2 and visit 3 samples using a commercially available kit following the manufacturer’s instructions (Sigma-Aldrich, # MAK116, St. Louis, MO).
Muscle Protein Isolation
Snap-frozen muscle samples (~30 mg) were homogenized following a 15 minute pre-incubation in 6 ul/mg muscle of ice cold lysis buffer with phosphatase inhibitors (Milliplex, Billerica, MA, USA; #43-040) with AEBSF protease inhibitor added (Milliplex; #101500) and then centrifuged at 14,000xg for 2 x 20 min at 4°C and assayed for protein content using the bicinchoninic acid (BCA) technique with BSA as a standard (Bio-Rad, Hercules, CA). Supernatant was stored at -80°C until further analysis.
Muscle Inflammatory/Cell Signaling Analysis
Twenty-five ug of total protein was resolved on 4-12% NuPAGE Bis-Tris gels (Novex, Life Technologies) and transferred overnight onto PVDF membranes (Bio-Rad, Hercules, CA). Immunoprobing was completed with antibodies from Cell Signaling Technologies (Danvers, MA). Antibodies against proteins known to be involved in the pro-inflammatory signaling cascade in muscle were used, including, TWEAK, TWEAK Receptor, Fn14, SOCS3, p50/p105 NFκβ, STAT3, phospho-STAT3 (Ser727), HSP27, and HSP70. HRP-conjugated secondary antibody (Pierce Thermo Scientific, Rockford, IL) was used at 1:2000 (w/v) followed by chemiluminescent detection. Bands were detected by chemiluminescence in a Bio-Rad (Hercules, CA) ChemiDoc XRS+ imaging system, and densitometry was performed using Bio-Rad analysis software.
Cytokine Protein Analysis
Muscle
Twenty ug of each homogenized muscle sample were loaded in duplicate onto plates for multianalyte profiling of cytokines using the Magpix (Luminex, Austin, TX) multiplex platform. Cytokines and soluble cytokine receptors (GCSF, IFNα2, IL10, IL13, IL15, IL1a, IL4, IL5, IL6, IL7, MCP1, GP130, IL6 Receptor, TNF Receptor 1, TNF Receptor 2) in muscle homogenates were measured using the MILLIPLEX® MAP assay kit (Millipore, #HCYTOMAG-60K) according to manufacturer’s specifications and analyzed using Milliplex Analyst software.
Plasma
Twenty-five ul of plasma from each sample were loaded in duplicate onto plates as above to determine multianylate profiling of specific cytokines (IL8, IL10, MCP1, TNFα).
Statistical Analysis
All data are expressed as means ± SEM unless otherwise noted. Student’s T-tests were used to analyze between group differences in subject descriptives. Outcome measures were analyzed using a 2 (groups) × 3 (times) repeated measures ANOVA. Main effects of treatment, time, and treatment–time interaction were determined by the method of Greenhouse–Geiser. If significant treatment by time interaction was detected, differences between and within treatments for specific times were analyzed with pairwise comparisons with significance set at p ≤ 0.05 after Bonferroni correction to account for multiple comparisons. Cohen’s effect size (d) was calculated to determine the magnitude of changes over time or between conditions and assessed as 0.2 = small effect, 0.5 = moderate effect, and 0.8 = large effect. Only changes with large effect sizes (where main effects were P > 0.05 < and P ≤ 0.10) are included in the results and discussion.
Results
Subjects included 24 volunteers (ages 60 – 79 years) who were enrolled in the study. One subject withdrew prior to visit 1 and a second subject withdrew immediately prior to visit 2, both for non-study related reasons. Data from these subjects has been excluded from analyses. The remaining 22 subjects completed the entire study (Table 1). No significant differences existed between groups or between pre- and post-supplementation physical characteristics.
Compliance, as measured by packet return (both empty and full), was over 97% with no single subject returning less than 94% of the packets. Diet records indicated no significant differences within groups over time or between groups for macronutrient composition (Supplementary Data Appendix A).
Dietary selenium pre-supplementation (60.3 + 38.5 ug/day) vs. post-supplementation (48.9 + 30.7 ug/day) within the blueberry group and dietary copper pre (1.0 + 1.2 mg/day) vs. post (0.7 + 0.4 mg/day) within the placebo group declined significantly (p < 0.05). No within or between group differences existed for any other micronutrients.
Cytokine and soluble cytokine receptor levels measured from the muscle biopsy sample were not significantly different between groups or within groups between visit 1 and visit 2, with one exception (Supplementary Data Appendix B). Muscle IL-10 levels were significantly higher at visit 1 in the placebo group compared to the blueberry group (p <0.05), but no differences existed post-supplementation or post-muscle stress at visits 2 or 3 between or within either group.
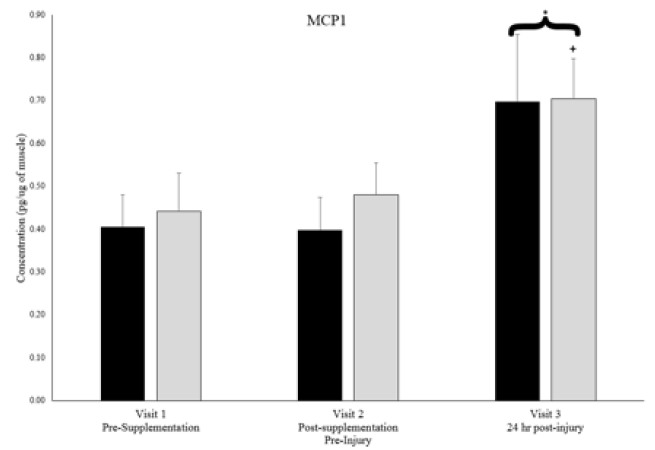
Plasma creatine kinase activity levels, an indicator of skeletal muscle injury, were 34.4 ± 8.3 units/L in both groups combined at visit 2 and significantly increased to 60.8 ± 10.0 units/L at visit 3, 24-hours post-muscle stress, an increase of 77%, however the magnitude of increase was the same in both groups. Similarly, from the muscle biopsy samples, monocyte chemotactic protein-1 (MCP-1) and TNFα Receptor 1 significantly increased from visit 2 (pre-muscle stress) to visit 3 (24 hr post-muscle stress) when groups were combined (Figures 2 & 3), but no between group differences existed. Soluble cytokine receptors including IL-6 receptor and TNFα receptors 1 & 2 were significantly higher at visit 3 in the placebo group than at visit 2, but no between group differences existed (Figures 2 & 3).
* denotes significant difference between timepoints 2 & 3 with groups combined. + denotes significant difference between timepoints 2 & 3 for respective group. P < 0.05.
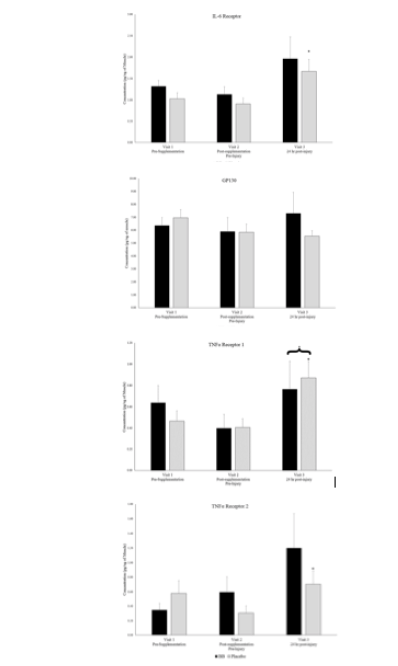
Figure 3
Muscle soluble cytokine receptor levels for blueberry (BB) and placebo groups at each timepoint.
* denotes significant difference between timepoints 2 & 3 with groups combined. + denotes significant difference between timepoints 2 & 3 for one group. P < 0.05.
As with the cytokines and receptors measured in the muscle, plasma cytokine and soluble cytokine receptor levels showed few differences due to blueberry supplementation (Supplementary Data Appendix C). Plasma IL-10 levels were significantly higher in the placebo group pre-supplementation at visit 1 (p < 0.05), but as in the muscle, no differences existed between groups at visit 2 or 3. Unlike the changes noted in the muscle due to the muscle stress stimulus in MCP-1 or the soluble cytokine receptors, no differences existed in plasma cytokines and soluble cytokines from visit 2 to visit 3 nor between groups at either visit.
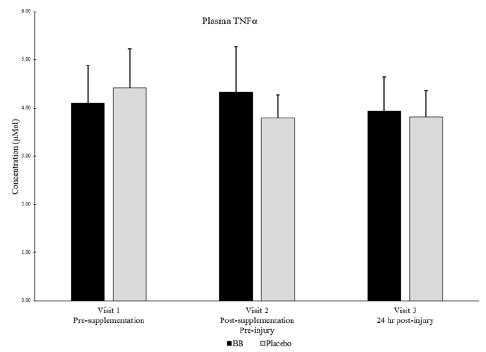
Of note, muscle levels of IL-6 were not significantly different between groups or within groups at any timepoint (Figure 4). Plasma levels of IL-6 were below detectable limits in most subjects at all timepoints. Muscle levels of TNFα were not detectable in any subjects at any timepoint, but plasma levels of TNFα were not significantly different between groups or within groups at any timepoint (Figure 5).
No significant differences between groups nor within subjects over time. P < 0.05. Note: Plasma IL-6 levels were undetectable
Western Blot analysis and multiplex cell signaling analysis of molecules involved in the IL-6 and TNFα skeletal muscle inflammation signaling cascade (SOCS3, NFkB, TWEAK, TWEAKR,) did not reveal any significant differences between groups nor within groups over time (Supplementary Data Appendix D). Additionally, no differences existed between or within groups in heat shock proteins 27, 60, 72, or 90a (Data not shown).
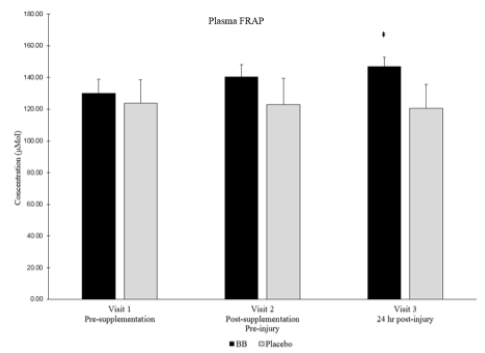
Total plasma antioxidant capacity as measured by FRAP was higher in the blueberry group than in the placebo group at visit 3 (Pearson’s Correlation p = 0.08, Cohen’s d = 0.85) (Figure 6). Plasma oxidative stress as measured by F2-isoprostanes was not different between groups or over time within groups.
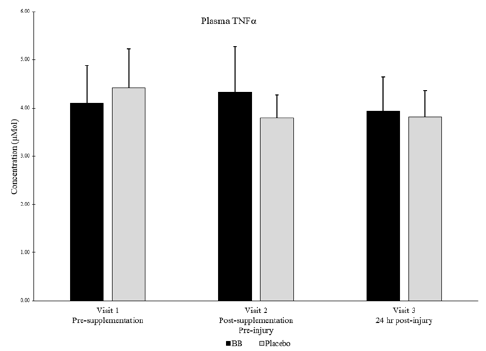
Figure 5
Plasma TNFα for blueberry (BB) and placebo groups at each timepoint. Note: Muscle levels of TNFα were not detectable.
No significant differences between groups nor within subjects over time. P < 0.05
‡ Denotes strong between group effect d > 0.8 & p < 0.10
Discussion
The findings that basal, non-stressed muscles have a similar inflammatory profile whether or not blueberries have been consumed daily for the previous six weeks and that the inflammatory response to a muscle stress stimulus is similar between blueberry and placebo groups disproves the original hypothesis. Some cytokine and signaling proteins were significantly different between blueberry and placebo groups prior to supplementation at visit 1, but most were no longer significantly different between groups after supplementation at visits 2 or 3. If differences between groups still existed, supplementation had no significant effect within subjects when compared between groups. While these results were unexpected because it was theorized that true group differences due to supplementation would be identified, six weeks of blueberry supplementation does not appear to have any clinically significant effect on the skeletal muscle inflammatory profile of older adults, nor on their regenerative response to exercise-induced injury as measured at the 24-hour time point post-injury.
Despite the lack of differences between blueberry and placebo groups in plasma cytokines, muscle cytokines and inflammation signaling proteins, a strong effect (Cohen’s d > 0.8 where main effects were between p > 0.05 and p < 0.10) was noted in total plasma antioxidant capacity as measured by FRAP. Total plasma antioxidant capacity was higher in the blueberry group post-muscle stress than in the placebo group. Muscle damaging exercise, such as eccentric exercise, is known to increase oxidant stress [9], and the increased antioxidant capacity noted in the current study is similar to what has been observed after blueberry consumption in runners (10) and young females after eccentric muscle stress (11). However, this is the first time this has been documented in older adults following muscle stress. The ultimate effects of blueberry consumption on inflammation levels and muscle recovery is difficult to determine though, as studies have determined no additional benefit of supplementing with different antioxidants such as Vitamin C or E (12), but others have shown beneficial effects on muscle but not against the inflammatory response (13). Further studies have proven that blunting the oxidant and/or inflammation response to exercise by consuming antioxidants might actually be detrimental and prevent the positive adaptations induced by exercise (14), so it is not clear what impact an increased systemic antioxidant capacity would have on regeneration in this aged population.
Unfortunately, most studies researching consumption of anti-inflammatories or antioxidants prior to exercise have not considered the impact of aging on these processes. That older adults have a blunted muscle regenerative response is well-established. The reasons this occurs are less clear, but, in vitro observations of muscle satellite cells from elderly subjects have proven that they have a decreased antioxidant capacity (15). Satellite cells are of incredible importance in determining the success of the muscle’s regenerative response, so improving the antioxidant capacity in this case, could be beneficial. Further insight can be gained by studies such as that of Trappe et al. in which daily supplementation with ibuprofen and acetaminophen were beneficial to older adults in a resistance training program and allowed for greater increases in muscle strength and size (5). Oxidative stress can certainly contribute to the type of inflammation that ibuprofen is attenuating, so it stands to reason that if consuming blueberries is increasing antioxidant capacity in the elderly, a beneficial effect to the exercised/damaged muscle could exist and muscle regenerative capacity could be improved.
Several factors might be contributing to our inability to discern some of the hypothesized effects of long-term blueberry consumption on muscle inflammation susceptibility in older adults:
1) Subjects might have been too healthy to experience much benefit from an anti-inflammatory food being added to an already healthy diet in an already healthy, and possibly non-inflamed body.
Utilizing prescription of hypertension medications as a surrogate for general health, 26% of subjects in this study were on prescription hypertension medications, much lower than the average of 53% – 63% of older Americans within the same BMI range as our subjects (16). Also of note in relation to overall health, subjects in the current study were well educated with 70% having completed college (67% of placebo vs 73% of blueberry) compared to approximately 30-40% of Americans in this age group (17). Since fruit and vegetable consumption generally increases with education level (18), it is likely, as 3-day diet records showed, that subjects in this study generally eat more fruits and vegetables than the average person, and their baseline anti-inflammatory food consumption might already be enough to prevent the traditional aging muscle inflammation susceptibility noted in previous research (3). An additional change to subject exclusion criteria to exclude potential subjects who consume any nutritional supplements, or at least provide a “wash-out” period prior to enrollment in the study might have affected the results. For the current study, only potential subjects who were taking medications or supplements known to affect inflammation were eliminated, otherwise, subjects were told not to modify their intake of diet or supplements for the duration of the study. Nutritional supplements have a wide variety of purported effects, many of which are untested, but the possibility exists that some were already on an anti-inflammatory nutritional regimen and as such, saw no benefit to further increasing anti-inflammatory capacity.
The possibility exists that subjects who are already experiencing inflammation at clinically high levels would be the most likely to benefit from an anti-inflammatory treatment. The subjects in the current study were very healthy 60+ year old men and women recruited similarly to a previous study in which systemic inflammation was not detectable, but muscle inflammation was higher than in younger adults (3). Over 75% of potential subjects were not enrolled due to numerous health factors that made them ineligible for the current study, although many were eliminated due to high body mass index (BMI). Since normal aging is associated with increased systemic inflammation (19), and our subjects likely did not have increased systemic inflammation compared to young adults, perhaps less stringent exclusion criteria to include those who have a higher BMI would have affected the results. Including subjects with higher BMI would likely have lead to inclusion of subjects with higher fat mass, which contributes to inflammation (20). Higher levels of inflammation, systemically and possibly in the muscle, could prove necessary for the clinical significance of blueberries anti-inflammatory properties to be realized.
2) The timing (24-hr post-stress stimulus) was not ideal to measure the true inflammatory insult.
Due to the need to limit the number of muscle biopsies each subject received, the only measurement post-muscle stress was 24 hours after the eccentric exercise bout. This timepoint was chosen for several reasons including the need to be able to compare results to previous studies, such as the original aged muscle inflammation susceptibility theory paper (3) which observed changes in inflammatory signaling pathways 24-hours post-muscle damage. Other markers of muscle damage are increased immediately post-exercise but also upwards of 48 hours post-exercise, such as MCP-1 levels (21, 22). Muscle soreness and plasma creatine kinase, both crude measures of muscle damage and inflammation, generally peak 24-48 hours post-damage (23). Cytokines on the other hand, are more difficult to determine with some, such as IL-6, often peaking after only a few hours, and sometimes returning back to normal within 24 hours or sometimes remaining elevated for upwards of 72 hours depending on the subjects and protocol (24-26). Due to these factors, for a single post-muscle stress biopsy, the 24-hour timepoint was deemed a timepoint likely to capture the most differences in both inflammation signaling within the cells as well as cytokine levels in plasma and muscle samples. However, given the obtained results, perhaps earlier and/or a later timepoints would have elucidated more of the changes.
3) Anti-inflammatory effects only work acutely, and since the subjects had been 24 hours without consuming blueberries when they experienced the muscle stress stimulus, the effects of blueberries on skeletal muscle had already expired.
Research on the consumption of another fruit product (cherry concentrate juice or powder) has shown a positive impact on muscle health in younger, well-trained subjects. Cherry concentrate consumption increased the functional recovery after eccentric exercise induced muscle damage and similar results were seen in a later study with a powdered form (6, 27). The supplements were given in the days leading up to the muscle damage and the day of and the days after the muscle damage occurred. This is an important difference to note from the current blueberry study, as subjects in the current study had not consumed blueberries in the 24 hours prior to the muscle damaging stimulus and did not consume any afterwards.
Measuring functional recovery was not the goal of the current study, but if in fact the beneficial effects of cherries on muscle recovery post-damage are due to anti-inflammatory properties of the cherries as was implied, it might be necessary that supplementation occur acutely, immediately before and after the injury. If this is the case, it is likely that blueberries would have a similar if not more potent effect. Blueberries have more of the anti-inflammatory anthocyanins than cherries (28), but the cherry juice concentrate was reported to have a higher amount of anthocyanins than raw cherries. However, this amount is still lower than the amount of anthocyanins consumed by subjects in the current study (547 mg/day for cherry juice vs. 3597 mg/day for blueberries).
4) The muscle stress stimulus was overwhelming, and perhaps not even a potent pharmacologic anti-inflammatory agent could prevent the response.
Finally, the possibility exists that the muscle stress stimulus caused by high volume eccentric contractions was overwhelming and the anti-inflammatory effects of blueberry supplementation over the previous 6-weeks was not enough to counteract such a strong inflammatory insult. As discussed, the single timepoint post-stress muscle biopsy makes this difficult to determine, but evidence exists that the inflammatory insult was present 24-hours after muscle stress. MCP-1 and TNFR1 levels were significantly higher post-muscle stress (visit 3) compared to pre-muscle stress (visit 2) when both groups were combined, and IL6R and TNFR2 levels were significantly higher in the placebo group post-muscle stress. While this was not the case in the blueberry group, receptor levels in the blueberry group were not statistically different from those in the placebo group post-muscle stress. These results indicate that the muscle is responding to the insult, but perhaps it was too much of an insult for blueberry supplementation to significantly counteract.
An interesting way to change the stimulus to determine if blueberry supplementation has any true effects on muscle health in older adults would be to use low “dose” stress stimuli as are typically seen in a normal resistance training program. Whereas the current study utilized 9 sets of 10 repetitions with a focus on the eccentric (muscle lengthening), damaging phase, a standard resistance training protocol has 2-3 sets of 10 repetitions focusing on a balanced concentric/eccentric movement. As was proven by Trappe et al., older adults consuming a pharmacologic anti-inflammatory agent such as ibuprofen over 12 weeks while partaking in a long-term resistance training program is beneficial for muscle gains in strength and hypertrophy (5). In the study, subjects were undergoing a relatively lower muscle stress three times per week in the form of resistance exercise training. This stimulus is generally accepted to cause an inflammatory response (29), but the observation that the ibuprofen administration improved muscle hypertrophy and strength, implies that the ibuprofen was able to prevent too large of an inflammation response. Muscle regeneration between exercise sessions might have been optimized due to the acute consumption of the anti-inflammatory agent concurrent with exercise training. A study modelled off the Trappe et al. study could be initiated to determine the effects of blueberries on the muscle health of older adults. A 12-week resistance training program with 4 groups; low dose blueberry, high dose blueberry, ibuprofen, and placebo, could compare the effects of a natural food like blueberries to a pharmacologic agent like ibuprofen and ultimately determine the clinical implications of blueberry consumption’s effects on the skeletal muscle inflammation susceptibility of older adults.
Long-term, daily blueberry consumption does not appear to have any effect, positive or negative, on the inflammatory profile of aged skeletal muscle, nor on the inflammatory response 24 hours after eccentric exercise induced muscle damage. Further research on the acute effects of blueberry consumption might elucidate mechanisms of inflammatory modulation of aged muscle as seen with other foods and pharmacologic agents, but daily consumption itself does not appear to cause inherent muscle inflammatory changes.
Acknowledgements: The authors would like to thank all research volunteers for their contributions as well as the many student volunteers who contributed their time helping make this project possible. This work was supported by funding from the United States Highbush Blueberry Association (E.K. Merritt) and the Appalachian State University Graduate Research Assistant Mentorship Award (D.A. Arminavage).
Author Contributions: E.K.M., L.S.M., and S.R.M. conception and design of research. All authors performed experiments. C.D, L.B., J.M.B., D.A., V.G., E.K.M., L.S.M., and S.R.M. analyzed data and interpreted results of experiments. C.D., V.G., and E.K.M. prepared figures, C.D, and E.K.M. drafted manuscript, C.D., E.K.M., L.S.M., and S.R.M. edited and revised manuscript. All authors approved final version of manuscript.
Conflict of interest statement: No conflicts of interest, financial or otherwise, are declared by the authors.
Ethical standard: This study was reviewed and approved by the Appalachian State University Institutional Review Board and complies with all laws of the U.S.A.
References
1. Conboy, I.M., M.J. Conboy, A.J. Wagers, E.R. Girma, I.L. Weissman, and T.A. Rando. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433(7027): 760-4.
2. Marsh, D.R., D.S. Criswell, J.A. Carson, and F.W. Booth. Myogenic regulatory factors during regeneration of skeletal muscle in young, adult, and old rats. J Appl Physiol. 1997;83(4): 1270-5.
3. Merritt, E.K., M.J. Stec, A. Thalacker-Mercer, S.T. Windham, J.M. Cross, D.P. Shelley, S. Craig Tuggle, D.J. Kosek, J.S. Kim, and M.M. Bamman. Heightened muscle inflammation susceptibility may impair regenerative capacity in aging humans. J Appl Physiol 1985; 115(6): 937-48.
4. Thalacker-Mercer, A.E., L.J. Dell’Italia, X. Cui, J.M. Cross, and M.M. Bamman. Differential genomic responses in old vs. young humans despite similar levels of modest muscle damage after resistance loading. Physiol Genomics. 2010;40(3): 141-9.
5. Trappe, T.A., C.C. Carroll, J.M. Dickinson, J.K. LeMoine, J.M. Haus, B.E. Sullivan, J.D. Lee, B. Jemiolo, E.M. Weinheimer, and C.J. Hollon. Influence of acetaminophen and ibuprofen on skeletal muscle adaptations to resistance exercise in older adults. Am J Physiol Regul Integr Comp Physiol. 2011;300(3): R655-62.
6. Bowtell, J.L., D.P. Sumners, A. Dyer, P. Fox, and K.N. Mileva. Montmorency cherry juice reduces muscle damage caused by intensive strength exercise. Med Sci Sports Exerc. 2011;43(8): 1544-51.
7. Johnson, S.A., A. Figueroa, N. Navaei, A. Wong, R. Kalfon, L.T. Ormsbee, R.G. Feresin, M.L. Elam, S. Hooshmand, M.E. Payton, and B.H. Arjmandi. Daily blueberry consumption improves blood pressure and arterial stiffness in postmenopausal women with pre- and stage 1-hypertension: a randomized, double-blind, placebo-controlled clinical trial. J Acad Nutr Diet. 2015;115(3): 369-77.
8. Benzie, I.F. and J.J. Strain. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239(1): 70-6.
9. Nikolaidis, M.G., A.Z. Jamurtas, V. Paschalis, I.G. Fatouros, Y. Koutedakis, and D. Kouretas. The effect of muscle-damaging exercise on blood and skeletal muscle oxidative stress: magnitude and time-course considerations. Sports Med. 2008;38(7): 579-606.
10. McAnulty, L.S., D.C. Nieman, C.L. Dumke, L.A. Shooter, D.A. Henson, A.C. Utter, G. Milne, and S.R. McAnulty. Effect of blueberry ingestion on natural killer cell counts, oxidative stress, and inflammation prior to and after 2.5 h of running. Appl Physiol Nutr Metab. 2011;36(6): 976-84.
11. McLeay, Y., M.J. Barnes, T. Mundel, S.M. Hurst, R.D. Hurst, and S.R. Stannard. Effect of New Zealand blueberry consumption on recovery from eccentric exercise-induced muscle damage. J Int Soc Sports Nutr. 2012;9(1): 19.
12. Theodorou, A.A., M.G. Nikolaidis, V. Paschalis, S. Koutsias, G. Panayiotou, I.G. Fatouros, Y. Koutedakis, and A.Z. Jamurtas. No effect of antioxidant supplementation on muscle performance and blood redox status adaptations to eccentric training. Am J Clin Nutr. 2011;93(6): 1373-83.
13. Silva, L.A., C.A. Pinho, P.C. Silveira, T. Tuon, C.T. De Souza, F. Dal-Pizzol, and R.A. Pinho. Vitamin E supplementation decreases muscular and oxidative damage but not inflammatory response induced by eccentric contraction. J Physiol Sci. 2010;60(1): 51-7.
14. Ristow, M., K. Zarse, A. Oberbach, N. Klöting, M. Birringer, M. Kiehntopf, M. Stumvoll, C.R. Kahn, and M. Blüher. Antioxidants prevent health-promoting effects of physical exercise in humans. Proceedings of the National Academy of Sciences. 2009;106(21): 8665-8670.
15. Fulle, S., S. Di Donna, C. Puglielli, T. Pietrangelo, S. Beccafico, R. Bellomo, F. Protasi, and G. Fano. Age-dependent imbalance of the antioxidative system in human satellite cells. Exp Gerontol. 2005;40(3): 189-97.
16. Usher, T., D.J. Gaskin, K. Bower, C. Rohde, and R.J. Thorpe, Jr. Residential Segregation and Hypertension Prevalence in Black and White Older Adults. J Appl Gerontol, 2016.
17. OECD. Adult Education Level. [Online] 2014 [cited 2016 February 24]; Available from: https://data.oecd.org/eduatt/adult-education-level.htm.
18. Lallukka, T., J. Pitkaniemi, O. Rahkonen, E. Roos, M. Laaksonen, and E. Lahelma. The association of income with fresh fruit and vegetable consumption at different levels of education. Eur J Clin Nutr. 2010;64(3): 324-7.
19. Singh, T. and A.B. Newman. Inflammatory markers in population studies of aging. Ageing Res Rev. 2011;10(3): 319-29.
20. Brinkley, T.E., F.C. Hsu, K.M. Beavers, T.S. Church, B.H. Goodpaster, R.S. Stafford, M. Pahor, S.B. Kritchevsky, and B.J. Nicklas. Total and abdominal adiposity are associated with inflammation in older adults using a factor analysis approach. J Gerontol A Biol Sci Med Sci. 2012;67(10): 1099-106.
21. Shanely, R.A., D.C. Nieman, K.A. Zwetsloot, A.M. Knab, H. Imagita, B. Luo, B. Davis, and J.M. Zubeldia. Evaluation of Rhodiola rosea supplementation on skeletal muscle damage and inflammation in runners following a competitive marathon. Brain Behav Immun. 2014;39: 204-10.
22. Deyhle, M.R., A.M. Gier, K.C. Evans, D.L. Eggett, W.B. Nelson, A.C. Parcell, and R.D. Hyldah. Skeletal Muscle Inflammation Following Repeated Bouts of Lengthening Contractions in Humans. Front Physiol. 2015;6: 424.
23. Baird, M.F., S.M. Graham, J.S. Baker, and G.F. Bickerstaff. Creatine-kinase- and exercise-related muscle damage implications for muscle performance and recovery. J Nutr Metab. 2012: 960363.
24. Ostrowski, K., C. Hermann, A. Bangash, P. Schjerling, J.N. Nielsen, and B.K. Pedersen. A trauma-like elevation of plasma cytokines in humans in response to treadmill running. J Physiol. 1998;513 ( Pt 3): 889-94.
25. Starzak, D.E., S.J. Semple, L. Smith, and A. McKune. Differing cytokine responses by ethnic groups to a bout of exercise-induced muscle damage: A preliminary report. J Sports Med Phys Fitness, 2015.
26. Smith, L.L., A. Anwar, M. Fragen, C. Rananto, R. Johnson, and D. Holbert, Cytokines and cell adhesion molecules associated with high-intensity eccentric exercise. Eur J Appl Physiol. 2000;82(1-2): 61-7.
27. Levers, K., R. Dalton, E. Galvan, C. Goodenough, A. O’Connor, S. Simbo, N. Barringer, S.U. Mertens-Talcott, C. Rasmussen, M. Greenwood, S. Riechman, S. Crouse, and R.B. Kreider. Effects of powdered Montmorency tart cherry supplementation on an acute bout of intense lower body strength exercise in resistance trained males. J Int Soc Sports Nutr. 2015;12: 41.
28. Wu, X., G.R. Beecher, J.M. Holden, D.B. Haytowitz, S.E. Gebhardt, and R.L. Prior. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. J Agric Food Chem. 2006;54(11): 4069-75.
29. Phillips, M.D., J.B. Mitchell, L.M. Currie-Elolf, R.C. Yellott, and K.A. Hubing, Influence of commonly employed resistance exercise protocols on circulating IL-6 and indices of insulin sensitivity. J Strength Cond Res. 2010;24(4): 1091-101.