K. Kinoshita1, S. Satake1,2, Y. Matsui3, S. Kawashima2, H. Arai1,2,4
1. Section of Frailty Prevention, Department of Frailty Research, Center for Gerontology and Social Science, National Center for Geriatrics and Gerontology, Obu, Japan; 2. Department of Geriatric Medicine, Hospital, National Center for Geriatrics and Gerontology, Obu, Japan; 3. Department of Orthopedics, Hospital, National Center for Geriatrics and Gerontology, Obu, Japan; 4. Director, Hospital, National Center for Geriatrics and Gerontology, Obu, Japan
Corresponding Author: Kaori Kinoshita, R.D., M.S. National Center for Geriatrics and Gerontology, 7-430 Morioka, Obu, Aichi, Japan 474-8511, TEL: +81-562-46-2311, FAX: +81-562-44-8518, E-mail address: kino4ta@ncgg.go.jp
J Aging Res Clin Practice 2019;8:1-6
Published online January 2, 2019, http://dx.doi.org/10.14283/jarcp.2019.1
Abstract
Objectives: To evaluate the effects of β-hydroxy-β-methylbutyrate (HMB) on muscle strength, physical performance, and muscle mass without additional exercise training in older adults with low physical function. Design: Randomized, controlled trial (Open-label study). Setting: Outpatients. Participants: 34 senior outpatients with low physical function who do not exercise regularly. Intervention: 2.4 g of HMB (3.0 g of calcium β-hydroxy-β-methylbutyrate [CaHMB]) per day was given for 60 days, and subjects in the control group were asked to engage in daily activities as normal. Measurements: Weakness or low function was defined by the Asian Working Group for Sarcopenia criteria, then the participants were assigned to the HMB group or the control group. All participants underwent several evaluations such as grip strength, the timed up and go test, the 5-times chair stand test (5CS), and skeletal muscle mass index by the bioimpedance method at baseline and at the end of intervention or control period. Results: An intragroup comparison of pre- to post-treatment values showed significant improvement in grip strength and the 5CS in the HMB group (grip strength: HMB, 16.6±6.1 kg to 18.2±6.4 kg, P=.001; control, 16.5±4.3 kg to 16.7±4.7 kg, P=.729; 5CS: HMB, 11.0 [8.8-13.0] s to 10.1 [8.5-12.6] s, P=.011; control, 11.1 [8.6-13.8] s to 10.0 [8.8-11.3] s, P=.246). Two-way repeated measures analysis of variance (ANOVA) used to compare the HMB and control groups showed a significant improvement in grip strength in the HMB group compared with the control group (P=.029). Conclusion: A supplementation of HMB without additional exercise may improve muscle strength in older patients with low muscle strength.
Key words: Randomized controlled trial, elderly, Asian Working Group for Sarcopenia, dynapenia, low muscle strength.
Introduction
One of the important cause of disability in the later lives is thought to be frailty. Frailty, characterized primarily by malnutrition and sarcopenia (a condition featuring loss of muscle mass with either muscular weakness or low physical performance) (1), is reversible (2), meaning that proper evaluation and intervention could bring improvement.
Maintaining skeletal muscle mass by consuming sufficient caloric content and protein and maintaining muscle strength through adequate exercise are effective ways to prevent malnutrition and sarcopenia. Food consumption generally induces protein synthesis and reduces protein catabolism in the skeletal muscles (3), but muscle synthesis in the skeletal muscles of older people appears to be less responsive to amino acids (4), which is called as anabolic resistance. To overcome such a condition, enough consumption of protein or essential amino acids is required, with leucine particularly playing a central role in protein synthesis in the body (5, 6).
The β-hydroxy-β-methylbutyrate (HMB) is a natural metabolic product of leucine (7), but only 5% of the leucine consumed is reportedly converted in the body to HMB. The HMB stimulates body protein synthesis (8) with anabolic effects more powerful than those of leucine (9) and may therefore have a potential effect on muscle growth and performance (10). These findings suggest that HMB may be effective in sarcopenia and dynapenia (11), but research in people with sarcopenia or poor physical performance is currently lacking. The findings of systematic reviews suggest that HMB consumption plus exercise may increase muscular strength in older persons, however there are no firm conclusions on the effects of HMB alone (12, 13). No randomized, controlled trial of HMB in sarcopenia or low physical function has been conducted under the Asian Working Group for Sarcopenia (AWGS) criteria (14).
To address this deficit, we decided to evaluate the effects of HMB consumption without additional exercise and within activities of daily living on physical performance of older people with low physical function.
Methods
Participants and informed consent
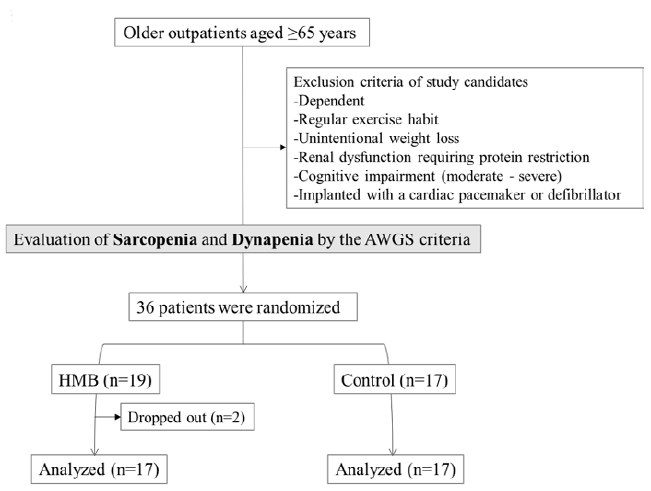
The participants were independent men and women aged 65 years and over who regularly visited the outpatient clinic of the department of geriatrics of the National Center for Geriatrics and Gerontology of Japan on an outpatient basis and were found to have weakness or low physical function according to the criteria of the AWGS (14) (Figure 1). Candidate participants were those who did not regularly exercise or undergo rehabilitation and had to be available for 2 months of the intervention/control period (the study period).
Candidates were excluded if they had experienced unintentional weight loss of 3 kg or more over the past 3 months, had an acute medical condition, had renal impairment requiring protein restriction, had moderate or greater cognitive impairment as shown by a Mini-Mental State Evaluation (MMSE) score of 18 or less, were certified as requiring assistance under Japan’s Long-term Care Insurance System, had a cardiac pacemaker, or were unsuited to physical performance evaluation because of visual or auditory impairment, quadriplegia, or a similar condition.
This study, which was grounded in the principles of the Declaration of Helsinki, was approved by the Ethics Committee of the National Center for Geriatrics and Gerontology of Japan. All participants were provided information about the purpose and procedures of the study and expected risks and benefits. Those who acknowledged the information and signed the informed consent form were enrolled.
Evaluation of low physical function
Weakness or low physical function was evaluated using the AWGS criteria (14). Grip strength and usual gait speed were first measured, and then muscle mass was evaluated. Men with a grip strength of less than 26 kg and women with a grip strength of less than 18 kg were considered to have muscular weakness. Participants with a gait speed of 0.8 m/s or below in a 5-m usual gait speed test (the middle 5 meters over an 11-meter walk) were considered to have decreased physical performance. A bioimpedance method was used to determine skeletal muscle mass. Men with a skeletal muscle mass index (SMI) of less than 7.0 kg/m² and women with an SMI of less than 5.7 kg/m² were considered to have low skeletal muscle mass. Sarcopenia was defined as low skeletal muscle mass and either muscle weakness or decreased physical performance. The low physical function was defined as either muscular weakness or decreased physical performance.
Randomization and intervention
Candidate participants who satisfied the criteria were examined by a physician and then informed about the study. Nutritional status was assessed with the Mini-Nutritional Assessment-Short Form (MNA®-SF) at the start of study period to confirm that none of the participants was malnourished. Those participants consenting to participate in the study were randomized by lottery to the intervention or control group.
The participants assigned to the interventional group were given 2 packets per day of a supplement containing 1.5 g of calcium HMB (1.2 g of HMB) (7 g of L-glutamine, 7 g of L-arginine, 1.5 g of calcium HMB; Abound™; Abbott Japan Co., Ltd., Tokyo) for 60 days. The participants were instructed to dissolve this powdered supplement in cold water before taking it because dissolving the supplement in hot water could have degraded its ingredients. The participants were given a calendar to use to record the amount consumed each day for 60 days.
The participants assigned to the control group were asked to conduct daily activities as normal for 60 days.
All participants underwent evaluations at baseline and at the end of study period (i.e., after 60 days). These evaluations were performed by a single trained nurse in the present study (Figure 1).
Participants were considered to have dropped out on becoming unable to undergo physical performance evaluations because of an acute illness, hospitalization, or injury during the study or when HMB compliance was less than 60%. This study was conducted in the full analysis set.
Outcome measures
All outcome measures were evaluated at baseline and at the end of study period. Grip strength was measured with a Smedley handgrip dynamometer (Matsumiya Ikaseiki Seisakusho Co., Ltd., Tokyo, Japan) facing outwards and the grip distance adjusted so that the second knuckle of the index finger was bent at a 90° angle. The participants were instructed to stand with their feet normally spaced and squeeze the dynamometer with their arm hanging so that the dynamometer did not touch their body or clothes. The grip strength of each hand was measured twice, with the highest measurement recorded.
The 5-times chair stand test (5CS) was used to evaluate leg strength. The participants, seated in a chair, were asked to stand and sit 5 times as quickly as possible with their arms folded in front of them. The time required was measured.
Skeletal muscle mass was measured with the Inbody 720 precision body composition analyzer (Inbody Japan Inc. Tokyo). Limb skeletal muscle mass (in kilograms) determined using the bioimpedance method was divided by the square of body height (in meters) to determine the SMI.
Functional mobility was evaluated with the timed up and go (TUG) test. The TUG test comprehensively evaluates functional mobility in terms of walking ability, dynamic balance, and agility. The time required for the participants to stand from a seated position, walk around a pylon 3 meters from the chair, return, and touch their pelvis to the chair was measured. The participants walked around the left and right sides of the pylon once. The shorter time was recorded. A time of 10 s or less is considered normal. Those with a time of 20 s or more are considered to require assistance in daily life (15).
Blood samples were taken to measure serum levels of IGF-1, DHEA-S, and 25(OH)D at baseline and at the end of the study period.
Sample size and statistical analyses
The required sample size was determined according to the calculations of a statistician. Based on the results of previous research (16), 2 groups of 17 participants each for a total of 34 participants were found to be necessary for a level of significance of 5% and power of 80% in statistical testing to evaluate the difference in the mean change in the primary outcome measure of grip strength.
SPSS 23.0 (IBM Japan, Tokyo, Japan) was used for all statistical analyses. A paired t-test was used to compare the pre- and post-treatment values in each group. Two-way repeated measures analysis of variance (ANOVA) was used to compare the changes between the groups. A t-test was used to compare mean differences from before to after treatment when a non-normal distribution was present. P-values less than .05 constituted a significant difference.
Results
The participants were sequentially randomized to the HMB group (n=19) and the control group (n=17). Two of the 19 participants in the HMB group were considered to have dropped out because they consumed less than the required amount of HMB (participant A: 54.2%, participant B: 50.0%). In the HMB group, 15 participants had sarcopenia. In the control group, 13 participants had sarcopenia.
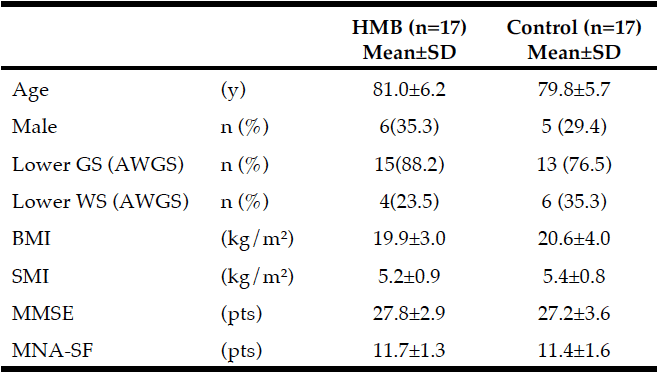
The baseline characteristics of the participants are shown in Table 1. Their mean age was 80.4±5.9 years. According to the AWGS criteria, 15 participants in the HMB group and 13 participants in the control group had decreased grip strength, and 4 participants in the HMB group and 6 participants in the control group had decreased gait speed. MNA®-SF scores were 11.7±1.3 in the HMB group and 11.4±1.6 in the control group. No participant in either group was malnourished.
Average intake of HMB in subjects: 2.21 g/day (2.76 g/day CaHMB); GS: grip strength, WS: walking speed, SMI: skeletal muscle mass index, AWGS: Asian Working Group for Sarcopenia, MMSE: Mini Mental State Examination, MNA®-SF: Mini Nutritional Assessment-Short Form
Mean HMB consumption was 2.21 g/day (7.6 g/day as CaHMB). Compliance was 92.1%.
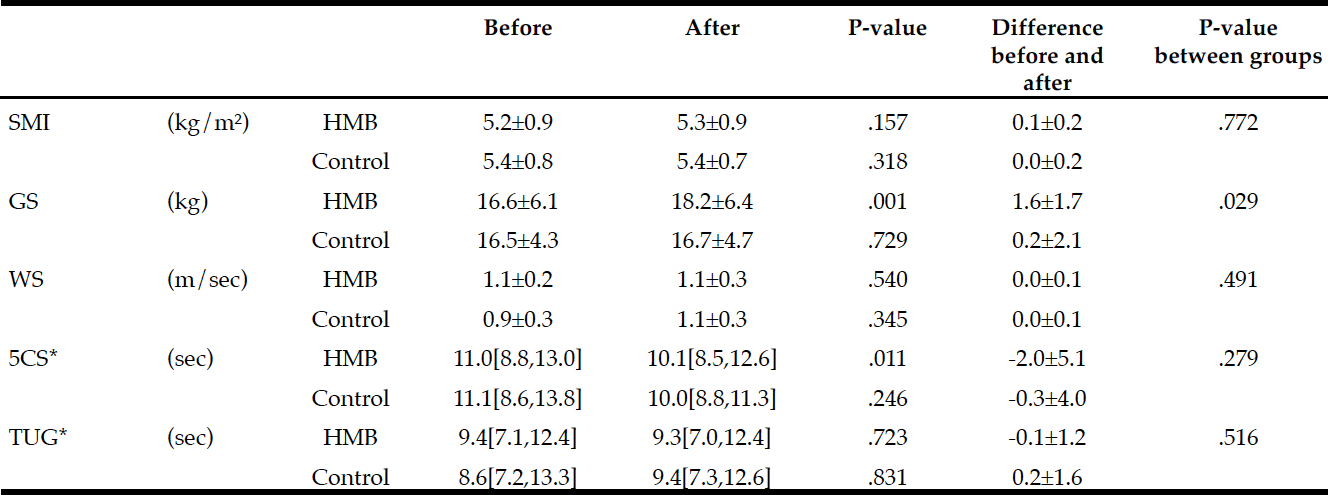
Changes in physical performance during the study period in each group are shown in Table 2. The HMB group achieved significant improvements in grip strength (P=.001) and 5CS (P=.011) with 60 days of intervention. SMI, however, did not change from before to after intervention. Gait speed and TUG scores as indicators of leg performance also showed no significant changes. No measure in the control group changed from before to after follow-up.
Intergroup comparisons of the changes using two-way repeated measures ANOVA showed a significant difference only in grip strength (P=.029).
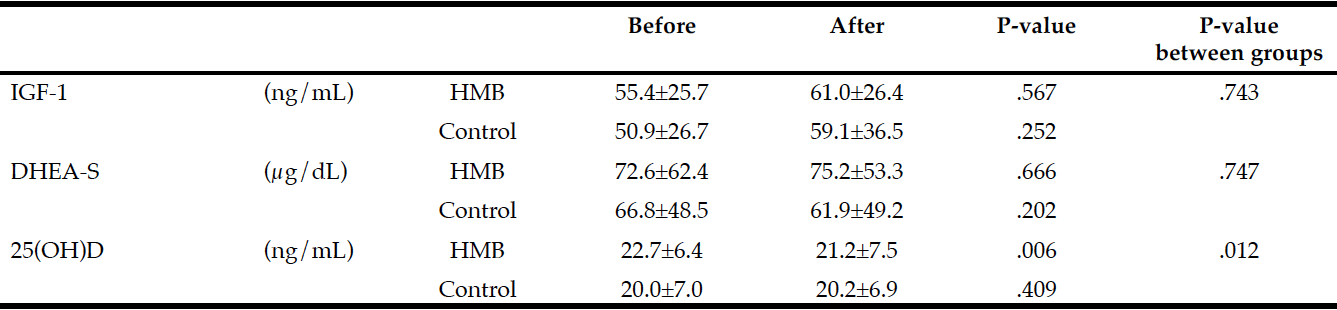
Changes in blood test values during the follow-up period in each group are shown in Table 3. The only significant difference from before to after treatment was seen in serum 25(OH)D levels, which were significantly lower after 60 days in the HMB group.
Any adverse events on HMB supplement intervention were not observed in this study.
Average intake of HMB in subjects: 2.21 g/day (2.76 g/day CaHMB); Difference within group : * Wilcoxon Signed-rank test, otherwise paired t-test; Difference between groups: * t-test, otherwise two-way repeated measures ANOVA; SMI: skeletal muscle mass index, GS: grip strength, WS: walking speed; 5CS: 5 times Chair Stand test, TUG: Timed Up and Go test
Average intake of HMB in subjects: 2.21 g/day (2.76 g/day CaHMB); Difference within group: Paired t-test; Difference between groups: Two-way repeated measures ANOVA
Discussion
This study evaluated the effects of intervention with 60 days of 2.4 g of HMB (3.0 g of CaHMB) consumption without additional exercise in older people with low physical function. Grip strength improved significantly in the HMB group compared with the control group.
This finding supports the findings of a previous study conducted by Flakoll and colleagues (16). In their study, free- and assisted-living older women (mean age: 76.7 years) who were assigned to the intervention group were given 2.0 g of HMB daily for 12 weeks. Grip strength improved significantly in the present study, which featured intervention for 60 days (about 8 weeks). The duration of intervention in the present study, however, may have been too short to capture the effects of intervention on physical performance and muscle mass. Flakoll and colleagues (16) observed significant improvements in grip strength and leg strength, as well as a tendency of improvement in lean mass, after 12 weeks of intervention. Another study of the effects of consuming 2.0 g of CaHMB daily for 1 year found a significant increase in skeletal muscle mass, and in participants whose blood 25(OH)D level was at least 30 ng/mL, a significant improvement was found in leg strength (17). In the present study, 5CS in the HMB group was significantly shorter after 60 days of follow-up, but it did not differ significantly compared with the control group. Physical performance of the legs depends not only on muscular strength, but also pain caused by motor disorders. Such effects were not considered when selecting the participants, which means that the effects of HMB consumption on leg strength may not have been accurately assessed.
Recent studies have found plasma HMB levels in older people to be positively correlated with grip strength and percent appendicular lean mass (18). Although HMB levels in the blood were not measured, increased blood levels of HMB resulting from regular HMB consumption of a certain amount may have contributed to the increase in grip strength that was observed, given that 82.4% of the participants had low skeletal muscle mass (sarcopenia).
Poor grip strength has been associated with negative outcomes in older people. A survey of hospitalized patients found higher hospitalization costs among patients with poor grip strength (19). Separately, grip strength was found to be significantly predictive of quality of life and the condition requiring assistance after 1 year (20). Known to be associated with several measures of muscular strength, grip strength was recently found to be associated with tongue strength, which contributes to chewing and swallowing function (21, 22). Poor chewing and swallowing function contributes to nutritional imbalance and low food consumption, which in turn contribute to sarcopenia and malnutrition. People whose decline in chewing and swallowing function has progressed such that they can no longer eat regular meals require the support of a caregiver to prepare special meals that they can chew and swallow. When further progression of malnutrition and sarcopenia is present, physical performance also decreases to a level at which further assistance is required. Under the assumption that the improvement in grip strength following HMB consumption that was observed is associated with improved tongue strength, consuming HMB may help prevent declines in chewing and swallowing function.
In this study, blood 25(OH)D levels were significantly lower in the HMB group after 60 days than in the control group. Vitamin D, which binds to receptors in the skeletal muscle, may play a central role in regulating the growth, differentiation, and myotube size of skeletal muscle cells (23). Fuller and colleagues (17) observed a significant improvement in leg strength associated with HMB consumption only in participants with a blood 25(OH)D level of 30 ng/mL or greater. This finding, taken together with the findings of the present study, suggests that synergy between HMB and vitamin D may have certain effects on skeletal muscle synthesis and muscular strength enhancement, but further analysis is required because no studies have considered this speculation.
Nevertheless, there is a limitation regarding the blood vitamin D levels measured in this study. Vitamin D is normally synthesized in the body through the action of ultraviolet light, but the effects of ultraviolet light in the present study are unknown, because the participants’ exposure to sunlight was not measured. Other study limitations include the facts that the control group was not given a placebo, leg strength may not have been accurately assessed because the effects of leg pain and other conditions were not considered when selecting the participants, and the 60-day period of intervention was short. Further research that addresses these limitations is needed to better characterize the effects of HMB on low physical function.
Conclusions
The findings of the present study suggest that daily consumption of 2.4 g of HMB (3.0 g of CaHMB) for 60 days (about 8 weeks) without additional exercise may improve muscular strength in older people with low physical function. Although the present study identified no gain in skeletal muscle mass or improvement in physical performance, and no association with blood vitamin D levels was found, it indicated that HMB may be a viable treatment for older adults with low physical function.
Acknowledgments: Satomi Furuzono and Yayoi Sakuraba kindly assisted with participant measurements and visit scheduling throughout the study, and the authors would like to express their gratitude to them. The authors also deeply appreciate the kind help of our managerial dietician colleagues Noriko Kojima, Kayoko Hattori, and Saki Tomita for working with the participants and supporting this work.
Author Contributions: Study concept and design: Kaori Kinoshita, Shosuke Satake, and Hidenori Arai. Acquisition of data: Kaori Kinoshita, Shosuke Satake, Yasumoto matsui, Shuji Kawashima, and Hidenori Arai. Analysis and interpretation of data: Kaori Kinoshita, Shosuke Satake, and Hidenori Arai. Drafting of the manuscript: Kaori Kinoshita. Critical revision of the manuscript for important intellectual content: Shosuke Satake, Yasumoto Matsui, Shuji Kawashima, and Hidenori Arai.
Conflicts of interest: This work was supported by grants from the Honjo International Scholarship Foundation and the Chukyo Longevity Foundation, and with funds from Abbott Japan Co., Ltd. The funders had no role in study design, data collection, analysis and interpretation, decision to publish, or preparation of the manuscript. Furthermore, none of the authors have any commercial or financial involvement in connection with this study that represent or appear to represent any conflicts of interest.
Ethical standards: This study has been approved by the research ethics committee of National Center for Geriatrics and Gerontology, Japan.
Funding Sources: Abbott Japan, Honjo International Scholarship Foundation, and Chukyo Longevity Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
1. Xue Q L, Bandeen-Roche K, Varadhan R, Zhou J, Fried L P. Initial manifestations of frailty criteria and the development of frailty phenotype in the Women’s Health and Aging Study II. J Gerontol A Biol Sci Med Sci, 2008; 63(9): 984-90.
2. Gill T M, Gahbauer E A, Allore H G, Han L. Transitions between frailty states among community-living older persons. Arch Intern Med, 2006; 166(4): 418-23.
3. Bolster D R, Jefferson L S, Kimball S R. Regulation of protein synthesis associated with skeletal muscle hypertrophy by insulin-, amino acid- and exercise-induced signalling. Proc Nutr Soc, 2004; 63(2): 351-6.
4. Volpi E, Mittendorfer B, Rasmussen B B, Wolfe R R. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J Clin Endocrinol Metab, 2000; 85(12): 4481-90.
5. Volpi E, Kobayashi H, Sheffield-Moore M, Mittendorfer B, Wolfe R R. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr, 2003; 78(2): 250-8.
6. Katsanos C S, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe R R. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab, 2006; 291(2): E381-7.
7. Van Koevering M,Nissen S. Oxidation of leucine and alpha-ketoisocaproate to beta-hydroxy-beta-methylbutyrate in vivo. Am J Physiol, 1992; 262(1 Pt 1): E27-31.
8. Wilkinson D J, Hossain T, Hill D S, et al. Effects of leucine and its metabolite beta-hydroxy-beta-methylbutyrate on human skeletal muscle protein metabolism. J Physiol, 2013; 591(11): 2911-23.
9. Giron M D, Vilchez J D, Salto R, et al. Conversion of leucine to beta-hydroxy-beta-methylbutyrate by alpha-keto isocaproate dioxygenase is required for a potent stimulation of protein synthesis in L6 rat myotubes. J Cachexia Sarcopenia Muscle, 2016; 7(1): 68-78.
10. He X, Duan Y, Yao K, et al. beta-Hydroxy-beta-methylbutyrate, mitochondrial biogenesis, and skeletal muscle health. Amino Acids, 2016; 48(3): 653-64.
11. Cruz-Jentoft A J. Beta-hydroxy-beta-methyl butyrate (HMB): From experimental data to clinical evidence in sarcopenia. Curr Protein Pept Sci, 2017.
12. Holecek M. Beta-hydroxy-beta-methylbutyrate supplementation and skeletal muscle in healthy and muscle-wasting conditions. J Cachexia Sarcopenia Muscle, 2017; 8(4): 529-541.
13. Wu H, Xia Y, Jiang J, et al. Effect of beta-hydroxy-beta-methylbutyrate supplementation on muscle loss in older adults: a systematic review and meta-analysis. Arch Gerontol Geriatr, 2015; 61(2): 168-75.
14. Chen L K, Liu L K, Woo J, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc, 2014; 15(2): 95-101.
15. Podsiadlo D,Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc, 1991; 39(2): 142-8.
16. Flakoll P, Sharp R, Baier S, et al. Effect of beta-hydroxy-beta-methylbutyrate, arginine, and lysine supplementation on strength, functionality, body composition, and protein metabolism in elderly women. Nutrition, 2004; 20(5): 445-51.
17. Fuller J C, Jr., Baier S, Flakoll P, et al. Vitamin D status affects strength gains in older adults supplemented with a combination of beta-hydroxy-beta-methylbutyrate, arginine, and lysine: a cohort study. JPEN J Parenter Enteral Nutr, 2011; 35(6): 757-62.
18. Kuriyan R, Lokesh D P, Selvam S, et al. The relationship of endogenous plasma concentrations of beta-Hydroxy beta-Methyl Butyrate (HMB) to age and total appendicular lean mass in humans. Exp Gerontol, 2016; 81: 13-8.
19. Guerra R S, Amaral T F, Sousa A S, et al. Handgrip strength measurement as a predictor of hospitalization costs. Eur J Clin Nutr, 2015; 69(2): 187-92.
20. Chan O Y, van Houwelingen A H, Gussekloo J, Blom J W, den Elzen W P. Comparison of quadriceps strength and handgrip strength in their association with health outcomes in older adults in primary care. Age (Dordr), 2014; 36(5): 9714.
21. Mendes A E, Nascimento L, Mansur L L, Callegaro D, Jacob Filho W. Tongue forces and handgrip strength in normal individuals: association with swallowing. Clinics (Sao Paulo), 2015; 70(1): 41-5.
22. Butler S G, Stuart A, Leng X, et al. The relationship of aspiration status with tongue and handgrip strength in healthy older adults. J Gerontol A Biol Sci Med Sci, 2011; 66(4): 452-8.
23. Girgis C M, Clifton-Bligh R J, Mokbel N, Cheng K, Gunton J E. Vitamin D signaling regulates proliferation, differentiation, and myotube size in C2C12 skeletal muscle cells. Endocrinology, 2014; 155(2): 347-57.