M. Naseer1, C.Fagerström1,2
1. Center of competence, Blekinge county council, Karlskrona, Sweden; 2. Department of health, Blekinge Institute of Technology, Karlskrona, Sweden.
Corresponding Author: Mahwish Naseer, Center of competence, Blekinge county council, SE-371 41 Karlskrona, Sweden, Phone: 00 46 (0) 738953144, mahwish.naseer85@gmail.com
J Aging Res Clin Practice 2016;inpress
Published online December 7, 2016, http://dx.doi.org/10.14283/jarcp.2016.125
Abstract
Objective: This study aimed to investigate the risk of malnutrition and to evaluate the psychometric properties of the Subjective-Objective Malnutrition Risk Assessment (SOMRA), SOMRA cut-offs and Swedish-Guidelines on Malnutrition Risk Assessment (SGMRA) for Swedish people aged ≥ 60 years. Setting: This study included both older people living at home and those in special housing. Participants: 1222 of the 1402 subjects aged ≥ 60 years who had participated in the baseline survey (2001–2003) as part of the ongoing National Study on Aging and Care-Blekinge (SNAC-B) were included because they had provided complete information on Mini-Nutritional Assessment (MNA). Measurements: The risk of malnutrition was estimated by the SOMRA, MNA, and SGMRA. To measure concurrent validity, the Receiver Operating Characteristics (ROC) curve, Cohen’s kappa (κ) and Spearman’s rank correlation coefficient rho (rs) were used. Youden’s index (J) was computed to assess the optimal cut-off on SOMRA. Cronbach’s alpha (α) was used to test reliability. Results: The risks of malnutrition measured by SOMRA, MNA and SGMRA were 6.5%, 8.6% and 20.9%, respectively. The risk was higher among older people living in special housing compared to those at home (p < 0.05). Different optimal cut-offs on SOMRA were observed for residents living at home (≥ 1) and those in special housing (≥ 3). Compared to SGMRA, the SOMRA and SOMRA cut-off ≥ 3 gave higher values for J (0.68, 0.81, and 0.84, respectively), κ (0.59, 0.77, and 0.84, respectively) and rs (0.64, 0.78, and 0.84, respectively) for the older people in special housing. The reliability for SOMRA was α = 0.71. Conclusion: The risk of malnutrition was higher among older people in special housing than among those living at home. For the people in special housing, the SOMRA and SOMRA cut-off ≥ 3 showed higher concurrent validity with MNA compared to the SGMRA, but not for older people living at home. SOMRA includes six items, takes less time to implement and is composed of both subjective and anthropometric measurements; therefore, it is suitable for use in special housing and/or clinical settings to identify the risk of malnutrition or the need for nutritional support.
Key words: Malnutrition, older people, psychometric properties, home-living, special housing.
Introduction
In older people, physiological changes due to age, such as muscle mass depletion, decreased ability to chew, and digestion problems (1), in addition to the decline in functional ability, such as reduced ability to cook or inadequate access to grocery stores, increase the risk of malnutrition (2). The risk of malnutrition contributes to adverse health outcomes like frailty (3), hospital admissions, longer stays at hospital (4), and pre-term mortality (3, 5). The increased risk of malnutrition with aging and demographic transition in aging suggests routine nutritional risk assessment to reduce the burden of malnutrition associated adverse health outcomes. However, the lack of a universally accepted nutritional risk assessment instrument hinders the implementation of routine nutrition risk screening (6, 7).
The studies on older Swedish people showed that 17% of those living at home (8), 27%–38% in special housing (9, 10) and 41% of those receiving home care services (11) were at risk of malnutrition. The higher risk of malnutrition in the special housing and those receiving home care services indirectly emphasises on the nutritional risk assessment at the community level, because the risk of malnutrition in the home living is often overlooked and gets worse over time (8). This ultimately leads to hospital and nursing homes admissions. As majority of the older population lives at their homes; therefore, nutritional risk assessment at the community level may reduce the risk of irreversible effects of malnutrition and burden on the nursing home admissions. Moreover, the higher risk for those in special housing (a person is offered care around the clock such as nursing homes, sheltered accommodation, or group accommodation; Swedish Association of local authorities and regions, 2009) and for those receiving home care services can be attributed to reduced functional ability and to more common poor health among special housing and home care residents (11). The risk rate may also depend on the instrument used for risk assessment and the population under investigation, e.g., eating ability as a risk assessment instrument will show a higher risk of malnutrition among adults in special housing compared to those living at home. This is because difficulty in eating is common among older people in special housing (9) compared to among those living at home.
Mini-Nutritional Assessment (MNA) is an 18-item nutritional assessment instrument recommended by European Society for Parenteral and Enteral Nutrition (ESPEN) for older people (6), but the MNA may be too long to be used in routine nutritional risk assessment (12). Therefore, Rubenstein et al. (12) proposed the MNA-SF based on six questions. However, if a patient is identified as being at risk of malnutrition according to the MNA-SF, the implementation of a full MNA is needed. The Swedish society for clinical nutrition and metabolism (SWESPEN) has given three guidelines (involuntary weight-loss, body mass index (BMI), and eating difficulties) that should be considered to assess the risk of malnutrition in the special housing and/or hospitalised patients (13). The Swedish Guidelines on Malnutrition Risk Assessment (SGMRA) are short and easy to implement, but the robustness of SGMRA is questionable, because it may over- or underestimate the risk of malnutrition (14). Therefore, Naseer and Fagerström (14) proposed a Subjective-Objective Malnutrition Risk Assessment (SOMRA) instrument by merging the guidelines of ESPEN for community dwelling (6) and SGMRA for hospital and special housing (13). Hence, SOMRA is based on the guidelines for nutritional risk assessment for both living at home and those in special housing; therefore, it may have better psychometric properties than SGMRA.
Kondrup et al. (6) stated a number of psychometric properties that should be considered when evaluating the usefulness of a nutritional risk assessment tool. These methods include predictive validity, content validity, reliability and practical use. The SOMRA shows predictive validity for quality of life (14) and mortality over 7 years (5). However, it remains necessary to evaluate the content validity and reliability of SOMRA and to discuss its practical use. Content validity refers to the comprehensiveness of a measuring instrument that means a risk assessment instrument includes all the relevant components of the problem that is being measured (6). SOMRA (14) included all the components of nutritional risk assessment proposed by ESPEN and SGMRA that may ensures the content validity of SOMRA. Another way to validate a newly designed instrument is to make a comparison of a new instrument with another well-validated instrument, e.g., MNA (7). This is called concurrent validity and the concurrent validity of SOMRA remains to be investigated.
The three objectives of this study were: 1) to investigate the risk of malnutrition according to SOMRA, MNA, and SGMRA among Swedish people aged ≥ 60 years, 2) to investigate the concurrent validity of the SOMRA, cut-offs on the SOMRA and the SGMRA with MNA among Swedish older people aged ≥ 60 years, 3) to evaluate the reliability in terms of internal consistency of SOMRA and SGMRA.
Materials and Methods
Study population
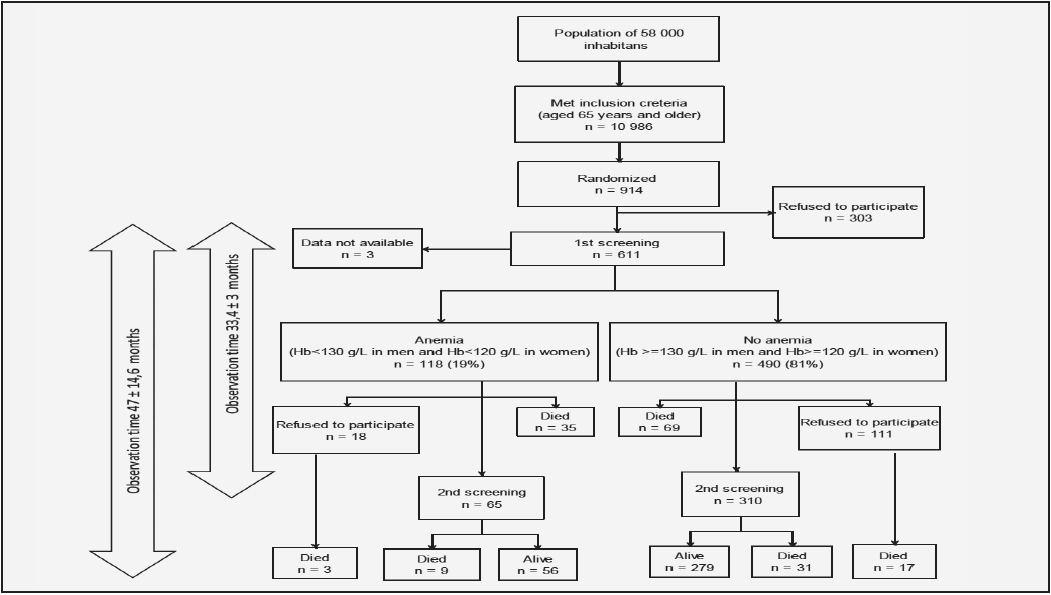
The sample for this study was drawn from an ongoing longitudinal cohort study: the Swedish National Study of Aging and Care (SNAC). The sample consisted of 1402 subjects aged 60–96 years, including both older people living at home (10.5% of them received home care services offered by municipality and 14.6% family support) and special housing residents (participants receiving 24-hour services/assisted living), who participated in the baseline survey (2001–2003) of SNAC-Blekinge (SNAC-B). The details of the SNAC are given elsewhere (15). The population was randomly selected for age cohorts of 60, 66, 72 and 78 years and the entire population was included for age cohorts 81, 84, 87, 90, 93 and 96 years. Participants were solicited through emails and phone calls, and reasons for non-participation were registered. Of the 1402, the participants who provided information on all items of the MNA (n=1222) were included in this study.
The data collection team consisted of nurses and physicians. The study was conducted in accordance with the Helsinki Declaration, and all participants provided written informed consent. The SNAC-B study was approved by the ethics committee of Lund University (LU 605-00, LU 744-00).
Measurements and instruments
A single-item self-administered questionnaire was used to collect information on age, gender, living arrangements, diseases (heart disease, diabetes, cancer, dementia and depression) and smoking. For subjects with dementia and depression, proxy measures were used. Economic status and physical activity were estimated by a two-item questionnaire. Functional ability was estimated by six items about activities of daily living (ADL; that is, bathing, dressing, toiletry, transferring, continence and feeding) (16) and by four items of instrumental ADL (IADL; that is, cleaning, transportation, shopping and cooking) (17). The score range was 0 to 6 for ADL and 0 to 4 for IADL. The score 0 represents independence and 1 represents dependence for all activities for ADL and IADL. Scores 6 and 4 represent total dependence for ADL and IADL, respectively. Thus, functional dependence was defined as ADL ≥ 1 and IADL ≥ 1 (5, 18).
Nutritional status as assessed by the MNA was used as the “gold standard”. MNA is a well-validated 18-item instrument (19). The MNA score dichotomized into “well-nourished” and “malnutrition / at risk of malnutrition” was used as outcome variable. The MNA was executed during a medical examination and anthropometric measurements were taken by the research staff. Body mass index (BMI) (kg/m2) was calculated from weight and height. Mid-arm circumference (MAC) and calf circumference (CC) were measured with a flexible tape to the nearest 0.1 cm of the left arm and leg, respectively (14).
SOMRA (14) includes three anthropometric measurements: BMI, MAC and CC, and three subjective measurements: decrease in food intake over the last 3 months, weight loss during the previous 3 months and eating ability, which refers to the subject’s ability to eat independently or only with help. The criterion for risk of malnutrition on the SOMRA was “the occurrence of at least one anthropometric measurement below cut-off, defined as the 15th percentile, (i.e., BMI < 22.7 kg/m2, MAC ≤ 25.5 cm and CC ≤ 32 cm) and the presence of at least one subjective measure (i.e., reduced food intake, weight loss and need for help when eating)”. In this study, cut-offs for BMI, MAC and CC were redefined as the 15th percentile due to the different sample size (n=1222) from that of the previous study (n=1402) (14). In addition, different cut-offs (≥ 1, ≥ 2, ≥ 3, ≥ 4, ≥ 5 & =6) for the 6 items of SOMRA were computed independently of whether measurement was subjective or objective. Thus, the SOMRA cut-off ≥ 1 was defined as the presence of at least one or more of these conditions: involuntary weight loss, decreased appetite, need for help when eating, BMI < 22.7 kg/m2, MAC ≤ 25.5 cm or CC ≤ 32 cm). The risk of malnutrition was also measured with respect to each cut-off of SOMRA.
The SGMRA (13) included 3 items; BMI < 20 if ≤ 69 years and BMI < 22 if ≥ 70 years; involuntary weight loss and eating difficulties. The presence of at least one condition out of these three delineated the risk of malnutrition.
Statistical analysis
Descriptive statistics are presented for the total population (n=1222) with respect to living arrangements (Table 1). For descriptive statistics, the mean and standard deviation (SD) were used for continuous variables and percentages were used for categorical variables. The data were not normally distributed; therefore, for comparison between groups (living at home vs in special housing), the χ2 test was used for categorical data and the Mann-Whitney U-test was used for ordinal and interval data. The concurrent validity of the SOMRA, the SOMRA cut-offs, and the SGMRA with MNA was tested by using three different statistical methods; Cohen’s kappa (κ), a receiver operating characteristic (ROC) curve, and Spearmen’s correlation coefficient (r). κ values represent the strength of agreement between the instruments. A κ value of <0.00 represents poor agreement and a κ value in the range of 0.81-1.80 shows almost perfect agreement (20). The strength of agreement is “slight” if κ = 0.00–0.20, “fair” if κ = 0.21–0.40, “moderate” if κ = 0.41–0.60, and “substantial” if κ = 0.61–0.80 (20).
An ROC curve is a graph of sensitivity and 1 – specificity, and the area under the ROC (AUROC) curve quantifies the diagnostic performance of a test variable at different cut-offs of the SOMRA (21). Sensitivity measures the ability to correctly identify cases and specificity measures the ability to correctly identify non-cases. Sensitivity and specificity were calculated from the ROC curve for the SOMRA, for various cut-offs of the SOMRA and for SGMRA. Sensitivity and specificity were considered equally important; therefore, Youden’s index (J = sensitivity + specificity – 1) was calculated to choose the appropriate cut-off (21). The Youden’s index (J) score ranges from 0 to 1, where 0 means that the test has no diagnostic value and 1 means that the test has perfect diagnostic value. In addition, positive predictive values (PPV) and negative predictive values (NPV) were calculated (21). PPV and NPV depend not only on specificity and sensitivity, but also on the prevalence of the health problem in question.
Reliability was tested in terms of internal consistency between the items of an instrument. The purpose of measuring internal consistency is to ensure that the instrument is measuring what it intends to measure (22). Cronbach’s alpha was used (23) to test the reliability; a coefficient score between 0.7 and 0.9 shows good reliability. To test the significance, a p-value < 0.05 was used. Analyses were conducted using SPSS statistical software, version 22 (SPSS Inc., Chicago, IL, USA) for Windows.
Results
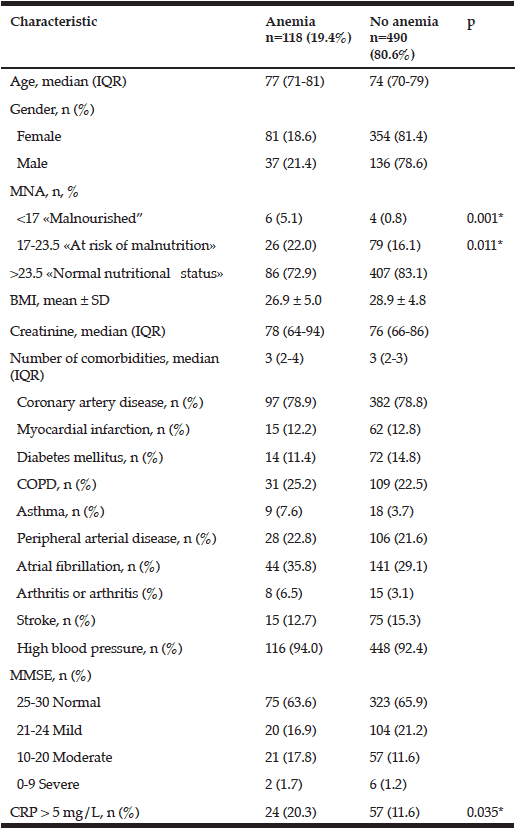
The mean age of the included study sample (n=1222) was 76.1 (SD, 10.0) years and 57.2% of the sample was female (Table 1). The prevalence of heart disease was highest (54.8%). The prevalence of the other illnesses was: diabetes (8.9%), cancer (13.7%), depression (12.6%), and dementia (2.7%). Compared to those living at home, the people in special housing were significantly older (81.7 years (SD 10.7) vs 75.7 years (SD 9.7)), had more dementia (13.6% vs 1.9%), were dependent according to the ADL (52.9% vs 14.9%) and the IADL measures (75.0% vs 44.5%). Moreover, the older people in special housing did significantly less light physical activity (44.7% vs 67.2%) compared to those living at home (Table 1).
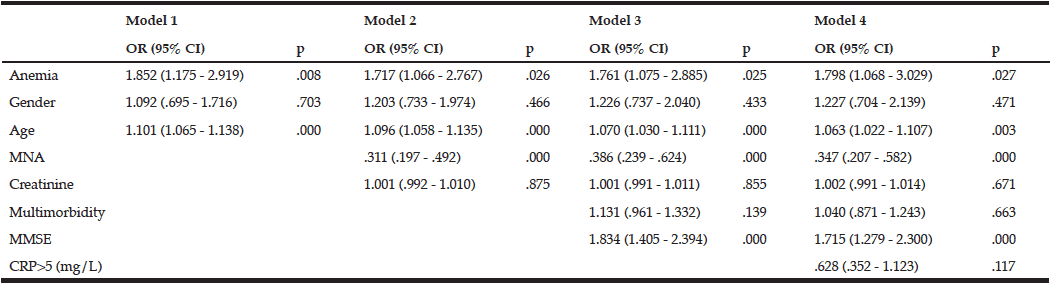
The risk of malnutrition as defined by various nutritional risk assessment instruments is given in Table 1. The MNA, SOMRA and SOMRA cut-off ≥ 3 showed almost similar levels of risk of malnutrition (8.6%, 6.5% and 9.3%, respectively). Moreover, the cut-off ≥ 2 on SOMRA (18.5%) and the SGMRA (20.9%) presented similar levels of risk of malnutrition. In the total population, the cut-off ≥ 1 on SOMRA showed the highest risk of malnutrition compared to other nutritional risk assessment instruments. However, the risk of malnutrition defined by various instruments except for the SOMRA cut-off ≥ 1 was significantly higher among the special housing residents than among older people living at home (Table 1).
The instrument’s validity measured by AUROC was 0.72 for SOMRA and 0.83 for SGMRA, irrespective of the living arrangement. Youden’s index values showed that the SGMRA had good validity compared to SOMRA (0.67 vs 0.45, respectively) in the total population. SOMRA was good for specificity (0.96), but not for sensitivity (0.49). However, the Spearman’s correlation co-efficient (rs) and kappa (κ) showed that SOMRA had moderate agreement with MNA than the SGMRA which has fair agreement with MNA (rs = 0.42 vs 0.39 and κ = 0.42 vs 0.30, respectively). In the total population, the optimal cut-offs on SOMRA as defined by Youden’s index were ≥ 1 and ≥ 2 , but correlation with MNA using kappa and Spearman’s correlation coefficient showed that the SOMRA cut-off ≥ 2 might be more valid than the SOMRA cut-off ≥ 1 (Table 2).
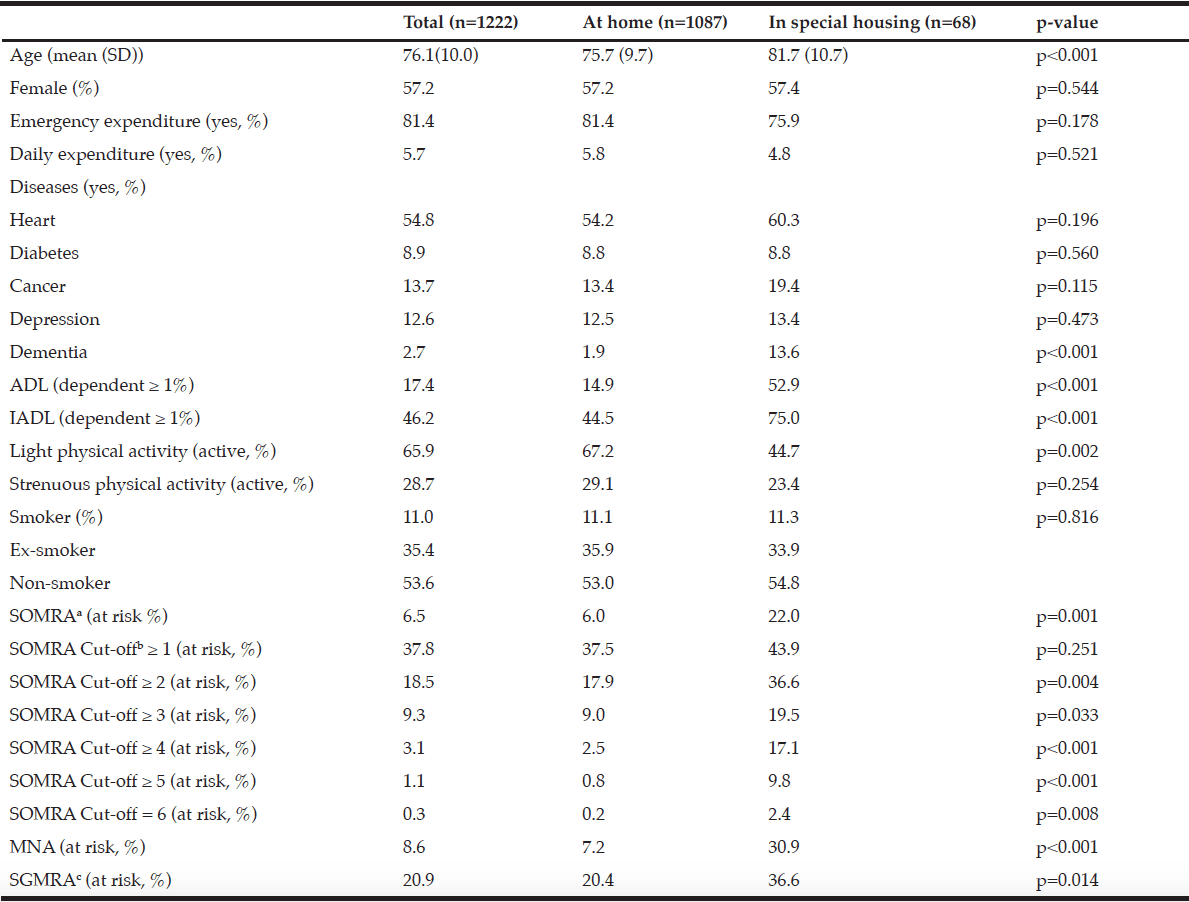
Table 1
Descriptive statistics for the total subject population (n=1222) stratified by housing arrangement
Abbreviations: SD, standard deviation; ADL, activities of daily living; IADL, instrumental ADL; SOMRA, Subjective-Objective Malnutrition Risk Assessment; MNA, mini-nutritional assessment; SGMRA, Swedish Guidelines on Malnutrition Risk Assessment.; Notes: The χ2 test was performed for nominal data and the Mann–Whitney U-test for the ordinal and interval data. p <0.05 was used to test significance. a. SOMRA: The risk of malnutrition was estimated by the occurrence of at least one anthropometric measure (BMI, MAC, CC) below cut-off, in addition to the presence of at least one subjective measure (declined food intake, weight loss, and eating difficulty); b. The SOMRA cut-offs were independent of subjective or objective criteria; c. The occurrence of at least one state: involuntary weight loss, body mass index (BMI) below a certain limit (< 20 if ≤ 69 years and < 22 if ≥ 70 years) or eating difficulties delineates the risk of malnutrition.
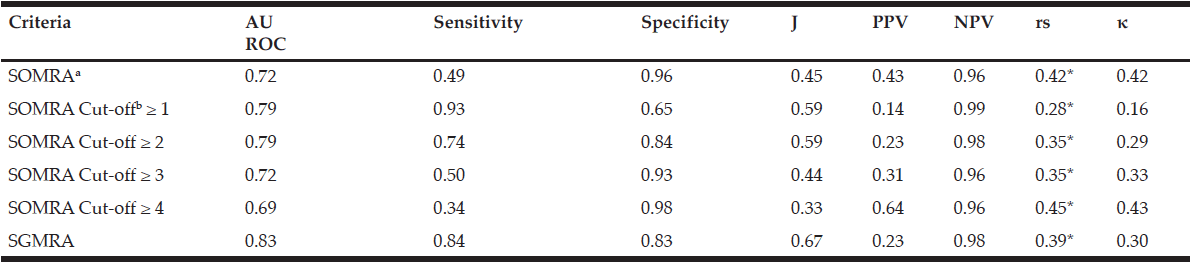
Table 2
Psychometric properties of the SOMRA, the SOMRA cut-offs and the SGMRA for the total population (n=1222)
Abbreviations: AUROC, area under the receiver-operating curve; J, Youden’s index; PPV, positive predictive value; NPV, negative predictive value; rs, Spearman’s correlation; κ, Cohen’s kappa; SOMRA, Subjective-Objective Malnutrition Risk Assessment; SGMRA, Swedish Guidelines on Malnutrition Risk Assessment; Notes: a. SOMRA: The risk of malnutrition was estimated by the occurrence of at least one anthropometric measure (BMI, MAC, CC) below cut-off, in addition to the presence of at least one subjective measure (declined food intake, weight loss, and eating difficulty); b. The SOMRA cut-offs were independent of subjective or objective criteria.* Correlation was significant at the 0.01 level.
For older people living at home, the SGMRA yielded a higher AUROC (0.83 vs 0.70) and Youden’s index value (0.66 vs 0.41) than did the SOMRA. The SOMRA cut-off ≥ 1 and the SGMRA produced almost the same Youden’s index values (0.60 vs 0.66); however, differences were observed for sensitivity and specificity. The SOMRA cut-off ≥ 1 showed higher sensitivity than the SGMRA (0.94 vs 0.83), but lower specificity (0.65 vs 0.83) for the people living at home. Moreover, the correlation with MNA using kappa for the SOMRA cut-off ≥1 was lower compared to the SGMRA (Table 3).
Abbreviations: AUROC, area under the receiver-operating curve; J, Youden’s index; PPV, positive predictive value; NPV, negative predictive value; rs, Spearman’s correlation; κ, Cohen’s kappa; SOMRA, Subjective-Objective Malnutrition Risk Assessment; SGMRA, Swedish Guidelines on Malnutrition Risk Assessment. Notes: a. SOMRA: The risk of malnutrition was estimated by the occurrence of at least one anthropometric measure (BMI, MAC, CC) below cut-off, in addition to the presence of at least one subjective measure (declined food intake, weight loss, and eating difficulty); b. The SOMRA cut-offs were independent of subjective or objective criteria; * Correlation was significant at the 0.01 level.
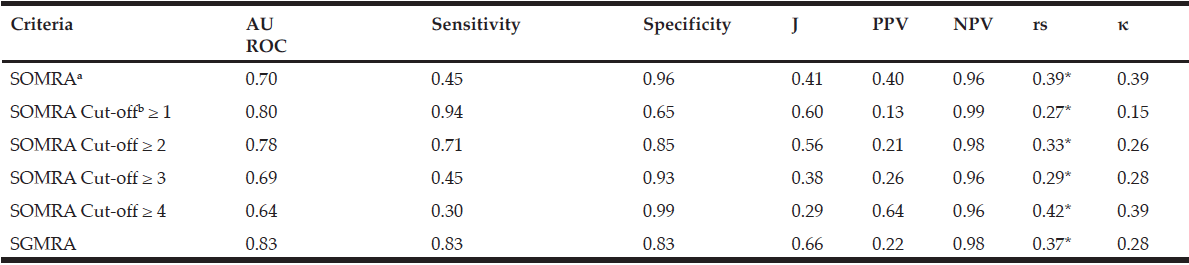
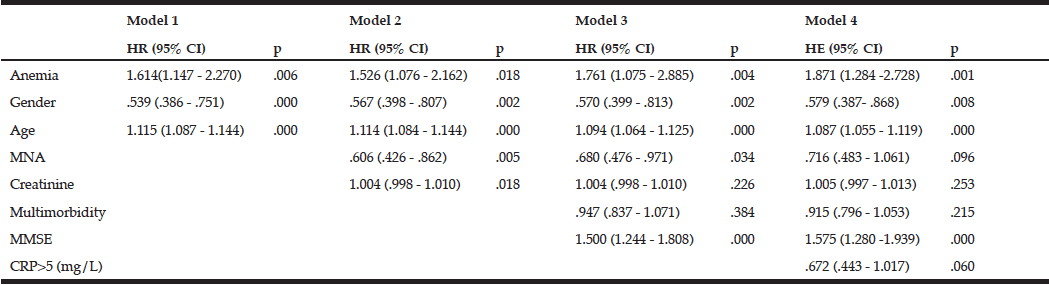
In special housing, SOMRA and SGMRA showed almost the same AUROC (0.90 vs 0.89), but SOMRA showed higher correlation with MNA (0.78 vs 0.64) and substantial agreement with MNA using kappa (0.77 vs 0.59) than SGMRA. In addition, the Youden’s index values showed that SOMRA had higher validity compared to the SGMRA (0.81 vs 0.68). Moreover, the SOMRA cut-off ≥ 3 was optimal for the special housing group and showed a slightly higher Youden’s index values than did the SOMRA (0.84 vs 0.81). The SOMRA and a SOMRA cut-off ≥ 3 showed the same sensitivity (0.87), but not the same specificity (0.97 vs 0.93). Using Spearman, the correlation was higher between MNA and a SOMRA cut-off ≥ 3 compared to between MNA and SOMRA (rs =0.84 vs 0.78). The agreement between MNA and SOMRA cut-off ≥ 3 was perfect while the agreement between MNA and SOMRA was substantial (κ =0.84 vs 0.77) (Table 4).
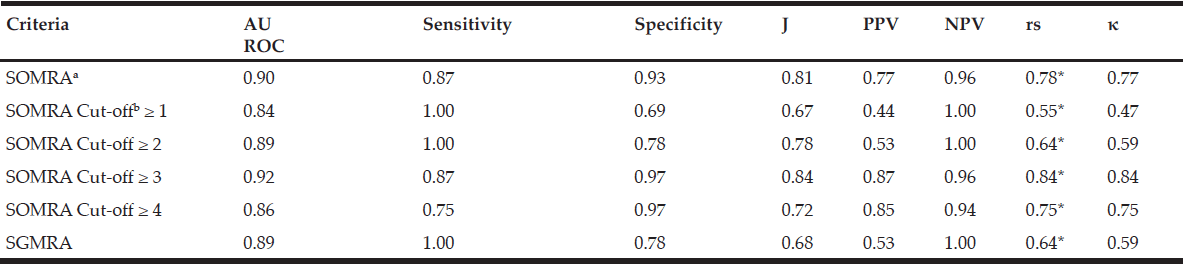
Table 4
Psychometric properties of SOMRA, SOMRA cut-offs and SGMRA for special-housing residents (n=68)
Abbreviations: AUROC, area under the receiver-operating curve; J, Youden’s index; PPV, positive predictive value; NPV, negative predictive value; rs, Spearman’s correlation; κ, Cohen’s kappa; SOMRA, Subjective-Objective Malnutrition Risk Assessment; SGMRA, Swedish Guidelines on Malnutrition Risk Assessment; Notes: a. SOMRA: The risk of malnutrition was estimated by the occurrence of at least one anthropometric measure (BMI, MAC, CC) below cut-off, in addition to the presence of at least one subjective measure (declined food intake, weight loss, and eating difficulty). b. The SOMRA cut-offs were independent of subjective or objective; * Correlation was significant at the 0.01 level.
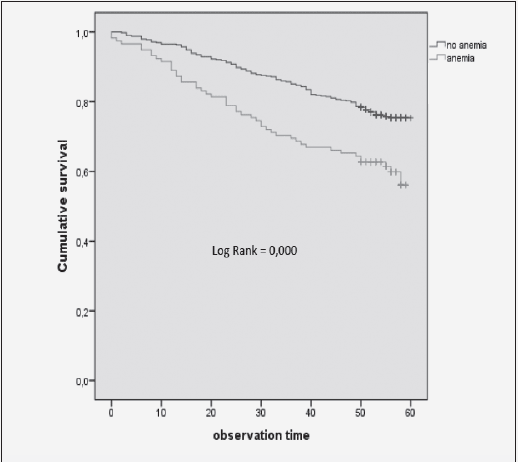
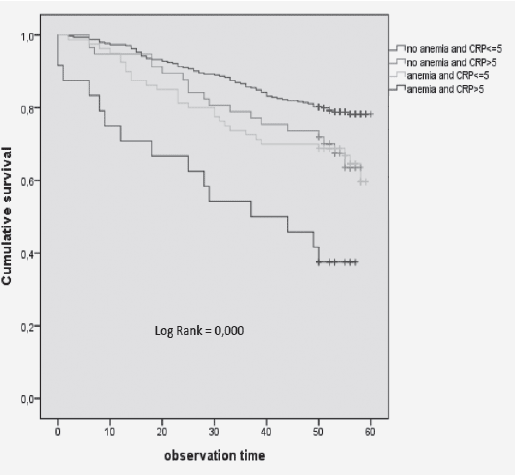
Furthermore, SOMRA showed good concurrent validity for the special housing group; therefore, a graphical illustration of the ROC curve is given. The cut-off ≥ 3 on SOMRA was optimal for the older people in special housing and gave a slightly higher AUROC curve (0.92 vs 0.90) compared to the SOMRA (Figure 1).
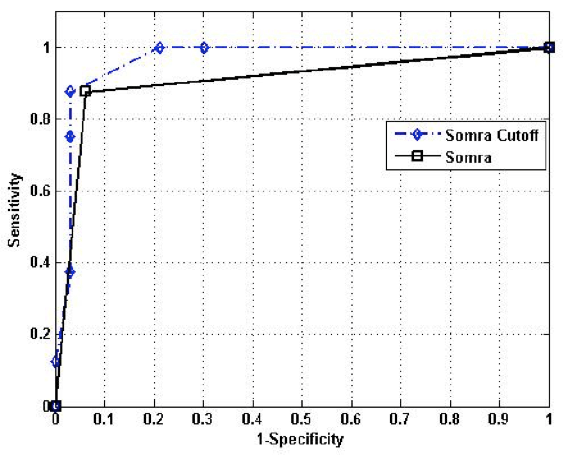
Figure 1
The receiver operative characteristics (ROC) curve for the SOMRA and the SOMRA cut-offs for Swedish people aged ≥ 60 years in special housing
The reliability (Cronbach’s alpha) for SOMRA based on six items was 0.71 that was higher than the SGMRA (α = 0.05) based on three items. The Cronbach’s alpha for SOMRA decreased to 0.50 if any of the anthropometric measurement out of six items deleted, and if any of the subjective measurement deleted the value of Cronbach’s alpha improved to maximum 0.74.
Discussion
The risk of malnutrition defined by the MNA, the SOMRA, and SGMRA was 8.6%, 6.5%, and 20.9%, respectively. In the total population, SOMRA showed good reliability, specificity, correlation and moderate agreement with MNA, but not sensitivity. For the older people in special housing, SOMRA showed substantial to perfect concurrent validity with MNA compared to SGMRA’s concurrent validity with the MNA, measured by various statistics; however, this was not true for older people living at home. The SOMRA cut-off ≥ 3 and the cut-off ≥ 1 were optimal for those in special housing and those living at home, respectively.
In this study, the risk of malnutrition was identified as being from 0.3% to 37.8%, according to various nutritional risk assessments. In the total population, the difference between the prevalence of risk of malnutrition reported for the MNA and SOMRA were less than for the SGMRA (8.6%, 6.5% and 20.9%, respectively). The risk of malnutrition defined by MNA and SOMRA in present study is similar with the findings of another study on Swedish older people living at home by using the MNA as a diagnostic instrument (risk = 7.4%) and baseline data collected during approximately the same years (1999-2001) (24) as the present study. However, the risk of malnutrition reported by SGMRA is lower than that reported in another Swedish study (27%) on special housing residents by using SGMRA as a risk assessment instrument (9). The instrument SGMRA is same in both studies but the difference in the risk rate is due to the different subject population. In present study, SGMRA reported highest risk rate than MNA and SGMRA. The SGMRA with three items may be more lenient compared to the SOMRA, which is based on an index developed from six variables (BMI, MAC, CC, reduced food intake, weight loss and need for help when eating). Therefore, in this study SGMRA may have overestimated the risk of malnutrition even though some subjects do not have the risk. This may be inferred from the specificity shown on the SGMRA and the SOMRA (0.83 and 0.96, respectively).
The findings of this study that the risk of malnutrition rate varies when different nutritional risk assessment instruments are used, even for the same subject population are consistent with the results of another study (7). The absence of universally accepted instrument for defining the risk of malnutrition limits the comparison between the prevalence of risk of malnutrition (25). Furthermore, it also makes it difficult to ascertain which instrument is more efficient at correctly defining the risk of malnutrition (7). An important assumption underlying nutritional risk assessment is that the person identified as being at risk of malnutrition will benefit from the risk being detected (12); therefore, it may be useful to detect which instrument efficiently predict the given outcome (7). Thus, future studies are needed to investigate which nutritional risk assessment instrument is most efficient in the prediction of various health outcomes.
The purpose of nutritional risk assessment is to identify the probability of developing or worsening malnutrition under the existing conditions, but the implementation of routine nutritional screening has often been hindered by the absence of universally accepted instrument for defining nutritional risk (6). However, Kondrup and colleagues (6) stated a number of psychometric properties that should be considered while designing or choosing the malnutrition risk assessment instrument, i.e., reliability, validity and practical use. Reliability coefficient shows the internal consistency between the items used in designing an instrument and demonstrates whether the results obtained from this collection of items are reliable (23). SOMRA based on six items showed greater reliability (α = 0.71) than SGMRA (α = 0.05) based on three items. These findings were likely as study sample of present study included both home living and those living in special housing and SOMRA (14) included items recommended for both home living and special housing older people. Interestingly, the reliability coefficient of SOMRA was almost same for home living (α = 0.70) and those living in special housing (α = 0.78); however, reliability coefficient of SGMRA was greater for special housing (α = 0.27) older people than those living in homes (α = 0.04). Thus, SOMRA is a reliable nutritional risk assessment instrument to be used in Swedish older people irrespective of their living arrangement.
Furthermore, for the concurrent validity with MNA, SOMRA showed good AUROC curve (0.72), specificity (0.96), a significant correlation (0.42), but poor sensitivity (0.49) for the total population. The failure to find higher values on Youden’s index in the total population could be due to different optimal cut-offs for those living at home (≥ 1) and those in special housing (≥ 3); also, the majority of the study sample was composed of older people living at home (94.1%). Moreover, MNA, the gold standard used in this study, is a nutritional assessment instrument developed for home care programmes, nursing homes and hospitals (6). The findings of this study that SOMRA has moderate agreement with MNA are similar to another study where Malnutrition Universal Screening Tool (MUST) showed moderate agreement with MNA-SF (7). This moderate agreement is likely, because MUST is a nutritional risk assessment tool recommended for the community dwelling while MNA is recommended for nursing homes and hospitals (6). Most of the study sample was comprised of home living; therefore, this might be the reason for not providing good concurrent validity of SOMRA with MNA for the total population. This can be deduced from the specific living arrangement analyses, in which SOMRA provided much better concurrent validity with MNA than did the SGMRA for the special housing group, but not for the group living at home. In sum, the comparison between various nutritional risk assessment instruments showed that SOMRA and the SOMRA cut-off ≥ 3 corresponded better with the MNA than did the SGMRA for the people in special housing. However, it remains uncertain which instrument is more robust for the older people living at home. Although the SGMRA showed fair concurrent validity with MNA for the people living at home, but SGMRA (13) and MNA are developed for special housing residents and hospitalized patients (6). Therefore, it may be hard to conclude whether these tools are equally useful for those living at home.
Kondrup et al. (6) stated that to evaluate the usefulness of a nutritional risk assessment instrument, it is also important that it should be practical, i.e., it must be rapid to administer, simple, and purposeful. The SOMRA (14) takes approximately 4 to 5 minutes to administer, compared to MNA, which takes approximately 10 to 15 minutes (19). Moreover, the SOMRA contains 6 items, compared to MNA, which is an 18-item tool; therefore, the SOMRA may place less of a burden on older people. Furthermore, the SOMRA (14) may be simple to administer because it does not require clinical instruments or doctor’s visits and can be administered by a nurse or assistant. However, instruments like a measuring tape and weighing scale are needed and it might be difficult to weigh and measure the length of a bed- ridden older person (12). Furthermore, the SOMRA needs to be performed by a nurse or staff, because it could be difficult for an older person to take MAC and CC measurements by him/herself.
Limitations of this study might include the response of nurses to the use of the SOMRA was not assessed. The sample size of special housing residents was small that may have influenced on the presentation of psychometric properties in the total population and with respect to the living arrangement. Although, the proportion of special housing residents and home living in the sample coincided with the national level of Sweden (26), but the study participants were generally healthier than non-participants. Approximately 10% refused to participate due to poor health; therefore, the risk of malnutrition might be under-represented. In addition, SGMRA used in this study for a comparison with SOMRA might be more clinical measurement in nature (α=0.05) instead an instrument for research purpose.
The strengths of this study include a large representative study sample and living arrangement specific analyses were executed to investigate the psychometric properties of SOMRA in home living and special housing residents. The SOMRA (14) includes three subjective and three anthropometric measures that can show a path from developing (past) to having (present) a risk of malnutrition. Among the subjective measures, inadequate dietary intake and the need for help when eating are indicators of future risk of malnutrition, particularly in older people. Moreover, involuntary weight loss emphasizes the need for routine (e.g., 3 to 6 months) follow-up for weight measurement to reduce the risk of a person becoming malnourished. Furthermore, the inclusion of three anthropometric measurements in the SOMRA may be useful for assessing the present nutritional status and they are more precise than the subjective measures; therefore, may reduce the chance of misclassifying the risk of malnutrition. The anthropometric measurements below cut-off emphasize the need for nutritional treatment/supplementation programmes to reduce the risk of nutrition associated poor health outcomes.
Conclusion
The risk of malnutrition identified by the SOMRA, MNA, and SGMRA was 6.5%, 8.6%, and 20.9%, respectively. These findings showed that various nutritional risk assessment instruments gave different percentages of risk for the same population. Future studies are needed to investigate the outcome-specific validity of each instrument. The observed optimal cut-offs of SOMRA were different for older people in special housing (≥ 3) and those living at home (≥ 1). For older Swedish people in special housing, the SOMRA showed substantial concurrent validity with MNA, the gold standard used in this study, compared to the SGMRA. Moreover, the SOMRA and the SOMRA cut-off ≥ 3 showed almost same substantial concurrent validity with MNA and could be used for older people in special housing. Furthermore, the SOMRA showed good reliability and should be simple to administer, easy to use and place less burden on the subject. The SOMRA may also be used in a clinical setting to suggest whether it is necessary to observe changes in weight over time or whether nutritional support is needed. Thus, SOMRA can be proposed as a reliable, valid and easy to administer nutritional risk assessment instrument for older people living in special housing.
Ethical standards: According to Swedish integrity and security law, informed consent is needed before gathering data on individuals from different sources. For the SNAC-B study, verbal informed consent was obtained for questionnaires and written informed consent was obtained for medical examination. The SNAC-B study was approved by the ethics committee of Lund University (LU 605-00, LU 744-00).
Acknowledgement: The Swedish National Study on Aging and Care, SNAC (www.snac.org), was financially supported by the Ministry of Health and Social Affairs, Sweden, and the participating county councils, municipalities and university departments. The study was also supported by the Blekinge Institute of Technology.
Conflict of interest: None.
References
1. Amarantos E, Martinez A, Dwyer J. Nutrition and quality of life in older adults. J Gerontol 2001; 56A (2): 54-64.
2. Kuczmarski MF, Kuczmarski RJ, Najjar M. Descriptive anthropometric reference data for older Americans. J Am Diet Assoc 2000; 100: 59-66.
3. Payette H. Nutrition as a determinant of functional autonomy and quality of life in aging: A research program. Can J Physiol Pharmacol 2005; 83: 1061-1070.
4. Visvanathan R, Macintosh C, Callary M, Penhall R, Horowitz M, Chapman I. The nutritional status of 250 older Australian recipients of domiciliary care services and its association with outcomes at 12 Months. J Am Geriatr Soc 2003; 57: 1007-1011.
5. Naseer M, Forssell H, Fagerström C. Malnutrition, functional ability and mortality among older people aged ≥ 60 years: a 7-year longitudinal study. Eur J Clin Nutr 2015; doi:10.1038/ejcn.2015.196
6. Kondrup J, Allison SP, Elia M, Vellas B, Plauth M. ESPEN guidelines for nutrition screening 2002. Clin Nutr 2003; 22(4): 415-421.
7. Stratton RJ, Hackston A, Longmore D, Dixon R, Price S, Stround M, King C, Elia, M. Malnutrition in hospital outpatients and inpatients: prevalence, concurrent validity and ease of use of the “malnutrition universal screening tool” (MUST) for adults. Br J Nutr 2004; 92: 799-808.
8. Johansson L, Sidenvall B, Malmberg B, Christensson L. Who will become malnourished? A prospective study of factors associated with malnutrition in older persons living at home. J Nutr Health Aging 2009; 13(10):855-861.
9. Westergren A, Lindholm C, Axelsson C, Ulander K. Prevalence of eating difficulties and malnutrition among persons within hospital care and special accommodations. J Nutr Health Aging 2008; 12(1): 39-43.
10. Wikby K, Ek AC, Christensson L. Nutritional status in elderly people admitted to community residential homes: comparisons between two cohorts. J Nutr Health Aging 2006; 10(3): 232-238.
11. Saletti A, Johansson L, Yifter-Lindgren E, Wissing U, Österberg K, Cederholm T. Nutritional status and a 3-year follow-up in elderly receiving support at home. Gerentol 2005; 51: 192 –198
12. Rubenstein LZ, Harker JO, Salva A, Guigoz Y, Vellas B. Screening for undernutrition in geriatric practice: developing the short-form mini-nutritional assessment (MNA-SF). J Gerontol Med Sci 2001; 56(6): M366-M372.
13. SWESPEN, Swedish Society for Parenteral and Enteral Nutrition. Nutritionsbehandling i sjukvård och omsorg (Nutritional treatment in care and service). In Swedish. http://www.swespen.se/documents/Nutritionshandboken.pdf Accessed 21 Feb 2014.
14. Naseer M, Fagerström C. Prevalence and association of undernutrition with quality of life among Swedish people aged 60 years and above: results of the SNAC-B study. J Nutr Health Aging 2015; 19(10): 970-979.
15. Lagergren M, Fratiglioni L, Hallberg IR, Berglund J, Elmståhl S et al. A longitudinal study integrating population, care and social services data. The Swedish National Study on Aging and Care (SNAC). Aging Clin Exp Res 2004; 16: 158-168.
16. Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged, the index of ADL: a standardized measure of biological and psychosocial function. JAMA 1963; 185: 914–919.
17. Åsberg KH, Sonn U. The cumulative structure of personal and instrumental ADL: a study of elderly people in a health district. Scand J Rehab Med 1988; 21: 171–177.
18. Aguero-Torres H, Fratiglioni L, Guo Z, Viitanen M, Strauss EV, Winblad B. Dementia is the major cause of functional dependence in the elderly: 3-year follow-up data from a population-based study. Am J Public Health 1998; 88:1452–1456.
19. Vellas B, Villars H, Abellan G, Soto ME, Rolland Y, Guigoz Y, Morley JE, et al. Overview of the MNA-its history and challenges. J Nutr Health Aging 2006; 10(6): 456-465.
20. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977; 33: 159-174.
21. Bewick V, Cheek L, Ball J. Statistics review 13: receiver operating characteristics curves. Critical Care 2004; 8(6): 508-512.
22. Bryman A. Social Research Methods. 2008. 3rd Ed. Oxford University Press Inc, New York.
23. Cronbach LJ. Coefficient alpha and the internal structure of tests. Psychometrika 1951; 16(3): 297-334
24. Salminen H, Sääf M, Johansson SE, Ringertz H, Strender LE. Nutritional status, as determined by the Mini-Nutritional Assessment, and osteoporosis: a crosssectional study of an elderly female population. Eur J Clin Nutr 2006; 60: 486–493.
25. Corish CA, Flood P, Kennedy, NP. Comparison of nutritional risk screening tools in patients on admission to hospital. J Hum Nutr Dietet 2004; 17: 133-139.
26. The National Board of Health and Welfare. Older-Health and Care The Second Half