S.G. Slezak1, K.B Mahoney1, E.N.Renna1, I.E. Lofgren2, F. Xu1, D.L. Hatfield1, M.J. Delmonico1
1. Department of Kinesiology, University of Rhode Island, Kingston, Rhode Island, USA, 02881; 2. Department of Nutrition and Food Sciences, University of Rhode Island, Kingston, Rhode Island, USA, 02881
Corresponding Author: Matthew Delmonico, 25 West Independence Way, Kingston, RI 02881,USA, delmonico@uri.edu, Phone: 401-874-5440
J Aging Res Clin Practice 2017;6:163-167
Published online August 31, 2017, http://dx.doi.org/10.14283/jarcp.2017.21
Abstract
Objectives: To evaluate the prevalence of sarcopenia in a sample of older, sedentary women using criteria from the European Working Group on Sarcopenia in Older People (EWGSOP), the International Working Group (IWG), and the Foundation for the National Institutes of Health Sarcopenia Project (FNIHSP). Design: Cross-sectional analysis. Setting and Participants: Community-dwelling women (n = 61) aged 71.9 ± 4.6 years (mean±SD) with a BMI 27.3 ± 6.0 kg/m2 who by self-report were healthy and did not exercise were recruited and evaluated for sarcopenia. Measurements: Height, weight, grip strength, gait speed, and appendicular lean mass (via segmental multi-frequency bioelectrical impedance analysis: SMF-BIA) were measured. Prevalence was reported using descriptive statistics and a Fisher’s exact test was used to analyze the distribution frequency of sarcopenia classification by different criteria. Results: In this sample 14.8% met EWGSOP criteria, 6.6% met FNIHSP criteria, and 3.3% met IWG criteria. There was a borderline significant difference in distribution frequency between EWGSOP and IWG classification criteria (p=0.053). Conclusion: The variation in sarcopenia prevalence depending on the diagnostic criteria used is consistent with previous research and there are borderline significant differences between classification criteria in this population. These data suggest the need for additional examination to determine current cut points for ALM measured by SMF-BIA, as well as which established definition of sarcopenia is appropriate for this population.
Key words: Sarcopenia, older, women, bioelectrical impedance analysis, appendicular lean mass.
Introduction
Sarcopenia is the progressive, naturally occurring loss of lean muscle mass that accompanies the aging process (1). Decreases in lean muscle mass have been associated with reduced physical function, osteoporosis, and loss of independence (2-4). The estimated sarcopenia related health care costs in 2000 were $18.5 billion, with $7.7 billion attributed to women, and costs continue to rise (5-8). Furthermore, US census population estimates project that by 2050 the amount of US adults over the age of 65 will double (9). The increasing healthcare costs and growing population present a serious public health problem and especially for older women as there are more women over the age of 65 (9, 10). Therefore, early detection and intervention methods are critical to alleviate the chronic effects of this condition in older women.
The prevalence of sarcopenia has previously been reported using different diagnostic criteria, and has ranged from 1-30% in samples of older community-dwelling women (11-13). However, lack of agreement among criteria presents challenges for clinicians and researchers attempting to identify sarcopenic individuals. Recently, three sets of diagnostic criteria for sarcopenia have been developed by the European Working Group on Sarcopenia in Older People (EWGSOP), the International Working Group (IWG), and the Foundation for the National Institutes of Health Sarcopenia Project (FNIHSP) (14-17). These criteria include measures of lean mass, physical function, and/or muscular strength. However, these criteria do not use consistent variables and cut points for quantifying lean mass and physical functioning, and lack overall agreement.
Few studies have reported the prevalence of sarcopenia in older community dwelling women using these three sets of diagnostic criteria. However, in 2014 Dam et al. conducted a comparison of EWGSOP, IWG, and FNIHSP sarcopenia classification criteria among the FNIHSP cohort and found large variations in prevalence depending on the classification criteria used (18). While that was a thorough investigation, participants were not recruited based on their physical activity levels and it is unclear if prevalence estimates will vary in a sedentary cohort. Therefore, the purpose of this study was to report and compare the prevalence of sarcopenia using EWGSOP, IWG, and FNIHSP criteria in a sample of older, sedentary, community-dwelling Rhode Island women.
Methods
Study Design and Participants
To evaluate sarcopenia prevalence, a cross-sectional analysis was performed among a sample of older, community-dwelling Rhode Island women who were recruited for an intervention trial through talks and posters at local community and senior centers, and through word of mouth. Initial screening was conducted via telephone interview to include women who were postmenopausal, aged 65-84 years, and by self-report were not involved in a regular exercise program or participation in physical activities outside of activities of daily living. Reasons for study exclusion included failure to provide informed consent, inability to speak and read English, diagnosed cognitive impairment, and the inability to safely engage in mild to moderate intensity exercise. Participants with recent major joint, vascular, abdominal or thoracic surgery were excluded. Participants who self-reported clinically diagnosed cardiovascular disease, pulmonary disease, or with an implanted pacemaker or defibrillator were excluded. Also, participants with uncontrolled diabetes, hypertension, or anemia were excluded. Any participants who reported medication changes within 3 weeks or changes to lipid lowering medication within 6 months were excluded. Trained study staff members performed all components of data collection.
Eligible participants read and signed informed consent and also completed a teach-back process, which required participants to explain learned information on the consent form back to a study staff member to ensure informed consent. Anthropometric data were then collected followed by tests to evaluate participants’ body composition, muscular strength, and gait speed. All aspects of this study took place in the Kinesiology Department on the campus of the University of Rhode Island, Kingston, Rhode Island, USA. This study was approved by the Institutional Review Board of the University of Rhode Island.
Anthropometrics
Height was measured without shoes to the nearest 0.1 cm using a Seca wall mounted stadiometer and body weight was measured without shoes to the nearest 0.1 kg using a Seca balance beam scale (Seca, Chino, CA). Height and weight were measured in duplicate and averages were used to calculate body mass index (BMI).
Body Composition
Whole and regional body composition was measured via segmental multi-frequency bioelectrical impedance analysis (SMF-BIA) using an Inbody 570 Biospace device (Biospace Co, Ltd, Korea) according to the manufacturer’s guidelines. Participants were asked to be fully hydrated, fasted for > 4 hours, and to void their bladder prior to the test. Appendicular lean mass (ALM) was calculated as the sum of lean mass in both arms and legs and expressed in kg. In accordance with EWGSOP and IWG criteria, ALM was adjusted for height expressed as meters squared, while according to FNIHSP criteria ALM was adjusted for BMI.
Muscular Strength
Isometric handgrip strength has been documented as a safe and effective method of predicting total body strength and future disability (19, 20). Muscular strength was measured via grip strength from a seated position using a Jamar Hydraulic Hand Dynamometer (J.A. Preston, Corp., Jackson, MS). Participants completed two trials per hand and the highest overall score from either hand (kg) was used for sarcopenia classification.
Gait Speed
Gait speed is an easily assessed measure that has been shown to be predictive of future disability (21). To evaluate gait speed, participants were instructed to walk a 4-meter distance at their normal walking pace (22). Two trials were completed and the fastest time (meters/sec) was used for sarcopenia classification.
Sarcopenia Classification
Sarcopenia was classified using EWGSOP, IWG, and FNIHSP criteria published previously (14-16, 18). These criteria are the most prominent among the literature; incorporate symptoms associated with sarcopenia, and have been shown to identify clinically relevant, sarcopenia-induced deficiencies in strength and physical function. The EWGSOP criteria utilize established stages of sarcopenia classification (presarcopenia, sarcopenia, severe sarcopenia), with low ALM/ht2 (< 5.67 kg/m2) and the presence of low gait speed (≤ 0.8 m/s) or low grip strength (< 20 kg) required to be considered sarcopenic. A severe sarcopenia classification requires low ALM/ht2, gait speed, and grip strength (14). Presarcopenia was defined as having low ALM/ht2 only. The IWG criteria utilizes a “yes/no” classification method, requiring individuals to be below established cut points of both gait speed (< 1.0 m/s) and ALM/ht2 (< 5.67 kg/m2) to be considered sarcopenic (15). The FNIHSP also uses established stages of sarcopenia classification: “weak with low lean mass and weak and slow with low lean mass.” In contrast to EWGSOP and IWG criteria, the FNIHSP uses ALM/BMI (< 0.512) to quantify lean mass, while also using differing cut points of gait speed (< 0.8 m/s) and grip strength (< 16 kg) (16). A “weak with low lean mass” classification required participants to be below cut points of ALM/BMI and grip strength, while a “weak and slow with low lean mass” classification required participants to be below cut points of ALM/BMI, grip strength, and gait speed. Participant data were collected and applied to these individual sets of criteria to determine the prevalence of sarcopenia within this sample.
Statistical Analysis
Descriptive statistics were used to report the baseline characteristics (means ± standard deviation) of the cohort and sarcopenia prevalence. A Fisher’s exact test was used to determine the distribution frequency of sarcopenia classification among the different sets of classification criteria. Significance was set at p ≤ 0.05. Statistical analyses were performed using SAS statistical software, version 9.3 (SAS Institute Inc., Cary, NC).
Results
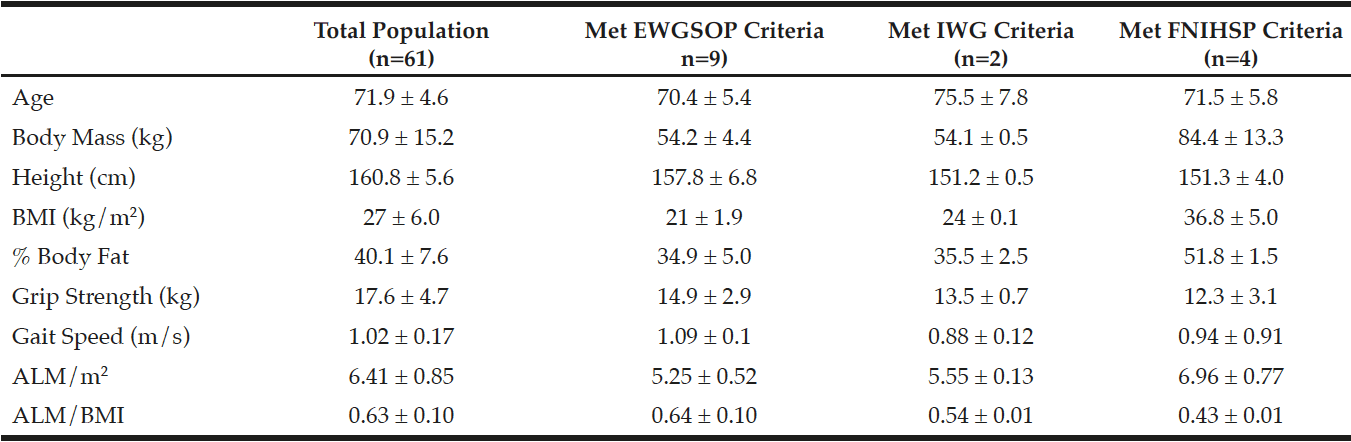
A total of 61 Caucasian women aged 71.9 ± 4.6 years were included in the analyses. Baseline characteristics of the population are presented in Table 1. Thirteen participants were considered sarcopenic. As shown in Table 1, nine (14.8%) participants were considered sarcopenic by EWGSOP criteria, four (6.6%) were considered weak with low ALM/BMI by FNIHSP criteria, and two (3.3%) participants were considered sarcopenic by IWG criteria. Sarcopenia prevalence for all criteria combined was 21.3% with no participant counted more than once. The two participants considered sarcopenic by IWG criteria were also considered sarcopenic by EWGSOP criteria. No other participants were considered sarcopenic by two or more sets of criteria. Additionally, no participants were considered pre-sarcopenic or severely sarcopenic by EWGSOP criteria or weak and slow with low lean mass by FNIHSP criteria. A Fisher’s exact test showed borderline significant differences in distribution frequency between EWGSOP and IWG classification criteria (p=0.053). No significant differences were found between other sets of classification criteria.
Data are presented as means ± standard deviations; Abbreviations: BMI = body mass index, ALM = sum of lean mass in both arms and both legs, m/s = meters per second, EWGSOP: European Working Group on Sarcopenia in Older People, IWG: International Working Group, FNIHSP: Foundation for the National Institutes of Health Sarcopenia Projet; Participants meeting EWGSOP criteria were sarcopenic (no pre-sarcopenia or severe sarcopenia); Participants meeting FNIHSP criteria had low lean mass and weakness (no low lean mass, weakness, and low physical function); Participants meeting IWG criteria (n=2) also met EWGSOP criteria and are included in that sample (n=9)
Discussion
These data indicate the large variation in sarcopenia prevalence depending on the classification criteria used. Within this sample, sarcopenia prevalence ranged from 3.3% to 14.8% with borderline significant differences in distribution frequency between EWGSOP and IWG criteria. This wide variation in prevalence is consistent with the findings of Cruz-Jentoft et al. (2014), who through systematic review found sarcopenia prevalence in community-dwelling women ranged from 1-30% when estimated using EWGSOP criteria (13). However, the authors expressed difficulty in comparing results of many studies due to inconsistent methodologies used in studies included in their review. In comparison, Patel et al. (2015) applied EWGSOP criteria to data from the Hertfordshire Cohort Study, which included 1,022 older women (23). While the baseline characteristics of that cohort closely resemble those of our sample, that study reported a 7.9% sarcopenia prevalence compared to our result of 14.8% using EWGSOP criteria. While those differences may be attributed to sample size, it may also be due to differences in grip strength. That study reported a mean grip strength of 26.3 kg while our results show a mean grip strength of only 17.6 kg, which is below the EWGSOP cut point for weakness in older women. This is consistent with the findings of Beaudart et al. (2014) who found grip strength criteria to largely influence sarcopenia prevalence (24). While there are considerably more data regarding sarcopenia prevalence using EWGSOP criteria, few studies have utilized IWG and/or FNIHSP criteria. However, Dam et al. in 2014 applied FNIHSP, IWG, and EWGSOP criteria to data collected from 2,950 older women through 9 different studies. That analysis found 2.3% of women to be weak and slow by FNIHSP criteria, 11.8% were sarcopenic by IWG criteria, and 13.3% were sarcopenic by EWGSOP criteria (18). Those researchers also noted that participants that had low lean mass by the ALM/BMI method were heavier with larger BMIs compared to those with low ALM/ht2. Our findings agree with those results, as every participant in our study who fell below the ALM/BMI cut-point had a BMI > 30 kg/m2. These results suggest that the FNIHSP criteria may be more effective at identifying sarcopenia in obese populations, while EWGSOP and IWG criteria may be more appropriate in non-obese populations. While our prevalence results vary with the findings of Dam et al. (18), possibly due to differences in sample size, it is evident that EWGSOP criteria consistently classify greater percentages of older women as sarcopenic when compared to FNIHSP and IWG criteria, and ALM adjusted for BMI may be the more effective method of identifying sarcopenia in obese, older women.
Reasons for variations in prevalence have recently been investigated by Masanés et al. (2016), who found that modification of EWGSOP lean mass cut points greatly varied sarcopenia prevalence, while modifying grip strength and gait speed cut points elicited little change in prevalence (25). However, those findings suggest that a large percentage of this population may have already been below the cut points for grip strength, as a combination of low ALM and weakness is required for a sarcopenia diagnosis by EWGSOP criteria.
Consequently our data show that the majority of participants considered sarcopenic by EWGSOP criteria had low ALM and weakness (n = 9), while no participants had low ALM accompanied with low gait speed. This also explains our low prevalence reported when using IWG criteria, which omits grip strength, and has a more liberal gait speed cut point. This suggests that inclusion of grip strength in sarcopenia diagnostic criteria may result in relatively higher prevalence estimates, and further screening for hand ailments (i.e. arthritis) may be necessary for accurate sarcopenia classification.
While the EWGSOP criteria are most prevalent within the literature, it does not take fat or body mass into consideration and may fail to classify those with sarcopenic obesity, as shown in our results (2). Moreover, the FNIHSP criteria may be ideal for the older female population as following menopause women typically experience increases in fat mass, which could prevent diagnosis by EWGSOP or IWG criteria (26). Our results underscore the discrepancies between different sets of sarcopenia classification criteria and therefore, inclusion of multiple sets of criteria may simplify the comparison of results and aid in determining population appropriate diagnostic criteria.
A small sample size, and a low number of participants who met classification criteria limited this study. A further limitation was the use of SMF-BIA to assess ALM rather than dual-energy x-ray absorptiometry (DXA). However, SMF-BIA has been found to be agreeable with DXA for measuring ALM in women, and BIA specific ALM/ht2 cut points presented by the EWGSOP were developed using prediction equations not applicable to the InBody 570 device (27, 28). Despite limitations, this study is novel in that EWGSOP, IWG, and FNIHSP criteria were all applied to the same sample of older, sedentary women from the same community. This allowed for the comparison of criteria without the need to adjust for sex, ethnicity, or activity levels. This study demonstrates the variability and limitations of current sarcopenia classification criteria, especially in obese individuals, and indicates the need for future research to develop current, criteria-appropriate cut-points for the measurement of ALM by SMF-BIA in this population to complement these findings.
Funding: This study was funded by the University of Rhode Island College of Human Sciences and Services.
Conflict of Interest: Matthew Delmonico has received research grants from the University of Rhode Island. All other authors report no conflicts of interest.
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
References
1. Morley JE, Baumgartner RN, Roubenoff R, Mayer J, Nair KS. Sarcopenia. J Lab Clin Med 2001;137:231-243.
2. Newman AB, Kupelian V, Visser M, Simonsick E, Goodpaster B, Nevitt M, Kritchevsky SB, Tylavsky FA, Rubin SM, Harris TB et al. Sarcopenia: alternative definitions and associations with lower extremity function. J Am Geriatr Soc 2003;51:1602-1609.
3. Batsis JA, Mackenzie TA, Barre LK, Lopez-Jimenez F, Bartels SJ. Sarcopenia, sarcopenic obesity and mortality in older adults: results from the National Health and Nutrition Examination Survey III. Eur J Clin Nutr 2014;68:1001-1007.
4. Delmonico MJ, Beck DT. The Current Understanding of Sarcopenia: Emerging Tools and Interventional Possibilities. American Journal of Lifestyle Medicine, 2015.
5. Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc 2004;52:80-85.
6. Antunes AC, Araújo DA, Veríssimo MT, Amaral TF. Sarcopenia and hospitalisation costs in older adults: a cross-sectional study. Nutrition & Dietetics, 2016.
7. Mijnarends D, Schols J, Halfens R, Meijers J, Luiking Y, Verlaan S, Evers S. Burden-of-illness of Dutch community-dwelling older adults with sarcopenia: Health related outcomes and costs. European Geriatric Medicine 2016;7:276-284.
8. Sousa A, Guerra R, Fonseca I, Pichel F, Ferreira S, Amaral T. Financial impact of sarcopenia on hospitalization costs. Eur J Clin Nutr 2016;70:1046-1051.
9. Administration on Aging, 2014. Projected Future Growth of the Older Population. U.S. Administration for Community Living. http://www.aoa.acl.gov/aging_statistics/future_growth/future_growth.aspx#age. Accessed 11/202016.
10. Borst SE. Interventions for sarcopenia and muscle weakness in older people. Age Ageing 2004;33:548-555.
11. Wen X, An P, Chen WC, Lv Y, Fu Q. Comparisons of sarcopenia prevalence based on different diagnostic criteria in Chinese older adults. J Nutr Health Aging 2015;19:342-347.
12. Tichet J, Vol S, Goxe D, Salle A, Berrut G, Ritz P. Prevalence of sarcopenia in the French senior population. J Nutr Health Aging 2008;12:202-206.
13. Cruz-Jentoft AJ, Landi F, Schneider SM, Zuniga C, Arai H, Boirie Y, Chen LK, Fielding RA, Martin FC, Michel JP et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014;43:748-759.
14. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412-423.
15. Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, Abellan van Kan G, Andrieu S, Bauer J, Breuille D et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc 2011;12:249-256.
16. Studenski SA, Peters KW, Alley DE, Cawthon PM, McLean RR, Harris TB, Ferrucci L, Guralnik JM, Fragala MS, Kenny AM et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci 2014;69:547-558.
17. McLean RR, Shardell MD, Alley DE, Cawthon PM, Fragala MS, Harris TB, Kenny AM, Peters KW, Ferrucci L, Guralnik JM et al. Criteria for clinically relevant weakness and low lean mass and their longitudinal association with incident mobility impairment and mortality: the foundation for the National Institutes of Health (FNIH) sarcopenia project. J Gerontol A Biol Sci Med Sci 2014;69:576-583.
18. Dam TT, Peters KW, Fragala M, Cawthon PM, Harris TB, McLean R, Shardell M, Alley DE, Kenny A, Ferrucci L et al. An evidence-based comparison of operational criteria for the presence of sarcopenia. J Gerontol A Biol Sci Med Sci 2014;69:584-590.
19. Laukkanen P, Heikkinen E, Kauppinen M. Muscle strength and mobility as predictors of survival in 75-84-year-old people. Age Ageing 1995;24:468-473.
20. Rantanen T, Guralnik JM, Foley D, Masaki K, Leveille S, Curb JD, White L. Midlife hand grip strength as a predictor of old age disability. JAMA 1999;281:558-560.
21. Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, Studenski S, Berkman LF, Wallace RB. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci 2000;55:M221-31.
22. Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994;49:M85-94.
23. Patel HP, White MC, Westbury L, Syddall HE, Stephens PJ, Clough GF, Cooper C, Sayer AA. Skeletal muscle morphology in sarcopenia defined using the EWGSOP criteria: findings from the Hertfordshire Sarcopenia Study (HSS). BMC Geriatr 2015;15:171-015-0171-4.
24. Beaudart C, Reginster JY, Slomian J, Buckinx F, Locquet M, Bruyere O. Prevalence of sarcopenia: the impact of different diagnostic cut-off limits. J Musculoskelet Neuronal Interact 2014;14:425-431.
25. Masanés F, Rojano iL, Salvà A, Serra-Rexach J, Artaza I, Formiga F, Cuesta F, López Soto A, Ruiz D, Cruz-Jentoft A. Cut-off points for muscle mass — not grip strength or gait speed — determine variations in sarcopenia prevalence. J Nutr Health Aging: 2016;1-5.
26. Ley CJ, Lees B, Stevenson JC. Sex- and menopause-associated changes in body-fat distribution. Am J Clin Nutr 1992;55:950-954.
27. Anderson LJ, Erceg DN, Schroeder ET. Utility of multifrequency bioelectrical impedance compared with dual-energy x-ray absorptiometry for assessment of total and regional body composition varies between men and women. Nutr Res 2012;32:479-485.
28. Mahoney K, 2016. Validation of bioelectrical impedance analysis for the measurement of appendicular lean mass in older women. Open Access Master’s Theses. Paper 845:http://digitalcommons.uri.edu/theses/845.