R. Zelig1,2, L. Byham-Gray1, S.R. Singer2, E.R. Hoskin3, A. Fleisch Marcus1, G. Verdino1, D.R. Radler1,2, R. Touger-Decker1,2
1. School of Health Professions’ Department of Nutritional Sciences at Rutgers University; 2. School of Dental Medicine, Department of Diagnostic Sciences, at Rutgers University; 3. School of Dental Medicine, Department of Restorative Dentistry, at Rutgers University
Corresponding Author: Rena Zelig, DCN, RDN, CDE, CSG, 65 Bergen Street #157, Newark, NJ 07107, (973)-972-5956, zeligre@shp.rutgers.edu
J Aging Res Clin Practice 2018;7:107-114
Published onlineAugust 7, 2018, http://dx.doi.org/10.14283/jarcp.2018.19
Abstract
Background and Objective: Older adults are at risk for both impaired oral health and suboptimal nutritional status. The objective of this study was to explore the relationships between malnutrition risk and missing teeth in community-dwelling older adults. Design: This was a retrospective cross-sectional analysis of data obtained from the electronic health records of 107 patients aged 65 and older who attended an urban northeast US dental school clinic between June 1, 2015 and July 15, 2016. Odontograms and radiographs were used to identify teeth numbers and locations; malnutrition risk was calculated using the Self-Mini Nutritional Assessment (Self-MNA). Relationships between numbers of teeth and malnutrition risk were assessed using bivariate logistic regression. Results: Participants (N=107) were 72.6 years (SD=5.6) of age; 50.5% were female. Mean Self-MNA score was 12.3 (SD=2.0) reflective of normal nutrition status; 20.6% were at risk for malnutrition, 4.7% were malnourished. Greater than 87% were partially or completely edentulous. Those with 10-19 teeth had lower Self-MNA scores (mean=11.6, SD=2.5) than those with 0-9 teeth (mean=12.7, SD=1.3) or 20 or more teeth (mean=12.6, SD=1.8) and had an increased risk for malnutrition (OR=2.5, p=0.076). Conclusion: The majority of this sample of older adults were partially edentulous and of normal nutritional status. Those with 10-19 teeth were more likely to be at risk for malnutrition. Further studies are needed to examine relationships between tooth loss and malnutrition risk and the impact of impaired dentition on the eating experience in a larger sample and to inform clinical practice.
Key words: Nutrition, Nutrition Assessment), MNA, Self-MNA, malnutrition, dentition, tooth loss, elderly.
Introduction
The relationships between nutrition and oral health are synergistic. The mouth is the entry way for food and fluid intake. If its integrity is impaired, the functional ability of an individual to consume an adequate diet may be adversely impacted. Older adults are a vulnerable population at high risk for both impaired oral health and malnutrition (1-4). Petersen (1) et al reported that 30% of older adults ages 65-74 globally were completely edentulous and many more were missing some of their natural teeth. Data from the United States (US) National Health and Nutrition Examination Survey (NHANES) 2011–2012, revealed that approximately 13% of those aged 65–74 and 26% of those aged 75 and over were edentulous (2).
Tooth loss may affect the ability of an individual to consume an adequate diet (5-9). Prior research has demonstrated relationships between nutritional status, nutrient intake, oral health and diet quality (5-9). The number and distribution of teeth impact masticatory function. Large population studies (5-9) have found that partial and complete edentulism are associated with changes in food and nutrient intake in older adults including decreased consumption of fruit, vegetables, dietary fiber, calcium, iron, and other vitamins. Independent of its cause, inadequate intake in older adults leads to weight loss, malnutrition, and ultimately increased morbidity and mortality (10, 11).
The etiology of malnutrition in older adults is multifaceted and may be related to physiological and psychological changes that contribute to variable food and nutrient intake (12-14). Kaiser and colleagues (3) pooled analyses from multinational studies which used the Mini Nutritional Assessment (MNA) to evaluate malnutrition prevalence and risk and reported that 22.8% of the older adults studied were malnourished and 46.2% were at risk for malnutrition. Huhmann et al (4) explored malnutrition risk in community dwelling older adults using the Self-MNA , a validated self-administered version of the MNA and found that 27% of subjects were malnourished, 38% were at risk of malnutrition, and 35% had normal nutrition status (4).
A systematic review by Zelig et al (15) exploring the associations between missing teeth and nutritional status (determined by MNA score) in community dwelling older adults revealed conflicting findings. Significant associations were found in five of eight studies between missing teeth (16-18) or the use of dental prostheses (19, 20) and malnutrition risk. MNA scores were significantly lower in those with fewer teeth/limited occlusion as compared to those with more teeth and/or more posterior occluding teeth pairs. However, other researchers did not report significant associations between occlusal status (21) or dental status (22) and MNA score.
Similarly, Toniazzo et al (23) systematically explored associations between malnutrition risk assessed by MNA or Subjective Global Assessment (SGA), and oral health status in elders and found that individuals with or at risk for malnutrition had significantly fewer teeth than those with normal nutritional status (23). However, similar to Zelig et al, a significant relationship between edentulism and malnutrition risk was not consistently identified.
Given the heterogeneity of the findings in this area (15, 23), the aim of this study was to explore the associations between nutritional status (as defined by the Self-MNA) and dentition status (missing teeth and edentulism with and without denture replacement) in older adults (=>65 years old) who came to the Rutgers School of Dental Medicine (RSDM) clinics in Newark, New Jersey (NJ), between June 1, 2015 and July 15, 2016. Analyses from the NHANES surveys conducted from 1999-2010 have consistently shown that partial and complete edentulism are more common in females, in persons who are Black or Hispanic, and in those who are of a lower socio-economic status (24, 25). The population of patients treated at the RSDM provided a convenience sample of vulnerable individuals at high risk for impaired dentition status and malnutrition given their racial diversity and low socio-economic status (24-26). We hypothesized that Self-MNA scores will be significantly lower in those with fewer teeth/limited occlusion as compared to those with more teeth and/or more posterior occluding teeth pairs (15, 23).
Materials and Methods
Sample Design and Sample Selection
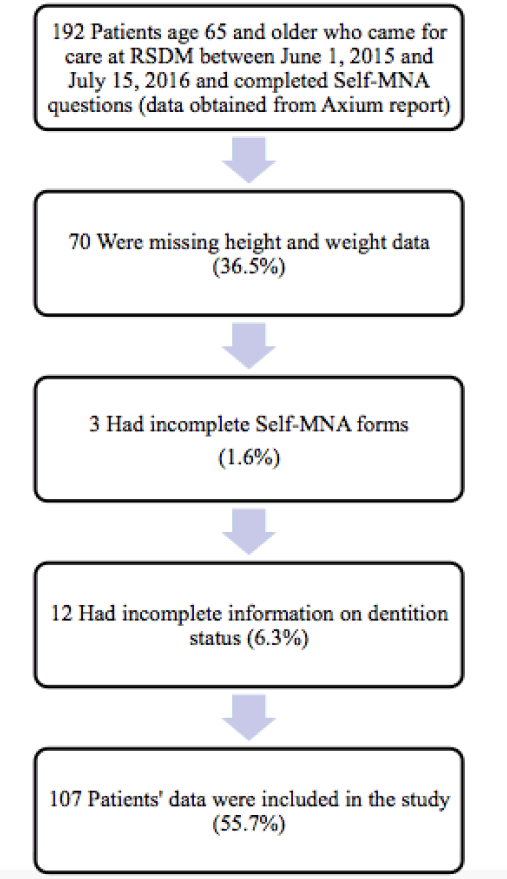
This was a retrospective cross-sectional analysis of data obtained from the electronic health records (EHR), (Axium, EXAN, Vancouver, BC, Canada) of patients who attended the RSDM clinic in Newark, NJ between June 1, 2015 and July 15, 2016. The EHR report contained select data provided by patients (n=192) during initial screening which included demographic characteristics, medical and dental history, and Self-MNA data. Patients were excluded (n=85) if they were younger than 65 years, height and/or weight were not available in the dental record, Self-MNA data were incomplete, and/or the number and location of teeth could not be accurately mined from the EHR. This study was approved by the Rutgers University Biomedical and Health Sciences Institutional Review Board.
Assessment of Nutritional Status
Data regarding nutritional status were obtained from the EHR using patient responses to the Self-MNA tool (Nestle Nutrition, available at http://www.mna-elderly.com/forms/Self_MNA_English_Imperial.pdf).
The original 18-item Mini Nutritional Assessment (MNA) was developed and validated in 1994 by Nestle Nutrition to detect risk for malnutrition in adults aged 65 and older (27). A condensed version of the MNA, the Mini Nutritional Assessment Short Form (MNA-SF), containing only six questions, was validated to further facilitate rapid nutritional screening in older adults, as it takes only three to five minutes to administer and retains the diagnostic accuracy of the full MNA (28, 29). In 2013, the Self-Administered MNA (Self-MNA) was adapted from the MNA-SF and validated by Huhmann et al (4) to allow the patient to provide a self-assessment of their nutritional status (4). The Self-MNA requires that the patient or their caregiver complete the assessment prior to their appointment with the healthcare provider; it can then be reviewed with the healthcare provider to identify risk factors for malnutrition (4). The Self-MNA contains six questions which address recent changes in intake and weight, mobility, recent stress, illness, dementia and sadness, as well as body mass index (BMI). A score of 0-7 is considered malnourished, 8-11 is at risk of malnutrition and 12-14 reflects normal nutritional status (4).
Assessment of Dentition Status
Number and location of teeth were mined from the EHR using patient digital radiography and odontogram data. A research assistant, trained and calibrated with a prosthodontist, recorded tooth numbers as either “present” or “missing”. If the tooth surface had been restored with a permanent crown, implant, or fixed bridge the tooth was reported as “present.” The four third molars/wisdom teeth were not included as they are not consistently present in all individuals. The presence and location of these permanent natural or restored tooth surfaces was also used to categorize the number of anterior and posterior occluding pairs of teeth (AOP and POP, respectively), defined as the “presence of a natural (or restored permanent) tooth on the maxilla and corresponding mandible, excluding remaining roots or root caps (page 316)” (30). Number of natural or restored teeth was categorized in a manner similar to prior research into those with 0-9 teeth, 10-19 teeth and 20 or more teeth (17, 31, 32).
Data Analysis
All statistical analyses were conducted using the Statistical Package for Social Sciences (version 22.0, SPSS Inc., Chicago, IL). A sample size of 85 was determined to be needed to establish if a correlation existed between the number of remaining teeth and Self-MNA score, based on a two-tailed test with an a priori alpha of p≤0.05 and 80% power to detect an small-moderate effect of at least 0.3 (33). Statistical significance was set at p<0.05.
Descriptive statistics were used to summarize all of the study variables. To test the hypothesis that Self-MNA scores will be significantly lower in those with fewer teeth / limited occlusion as compared to those with more teeth and/or more posterior occluding teeth pairs, Spearman’s Correlation Coefficient was used. Associations between categorical variables were evaluated using the chi-squared test; non-parametric Mann-Whitney and Kruskal-Wallis tests were used to compare groups. Simple logistic regression was used to assess the odds of being at risk for malnutrition or malnourished related to number of teeth present. To determine potential confounding by demographic characteristics, bivariate analyses were conducted in accordance to age, gender, race and ethnicity as well as prior medical history.
Results
Characteristics of the study sample
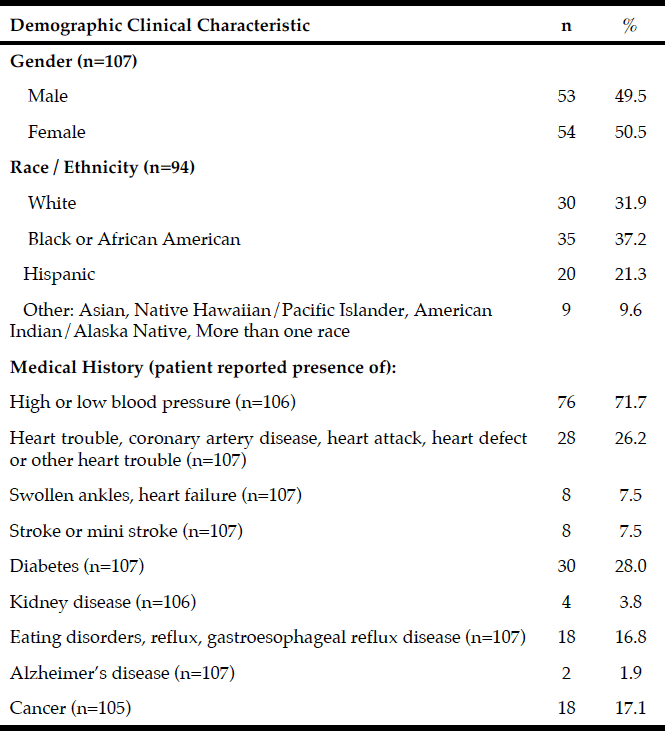
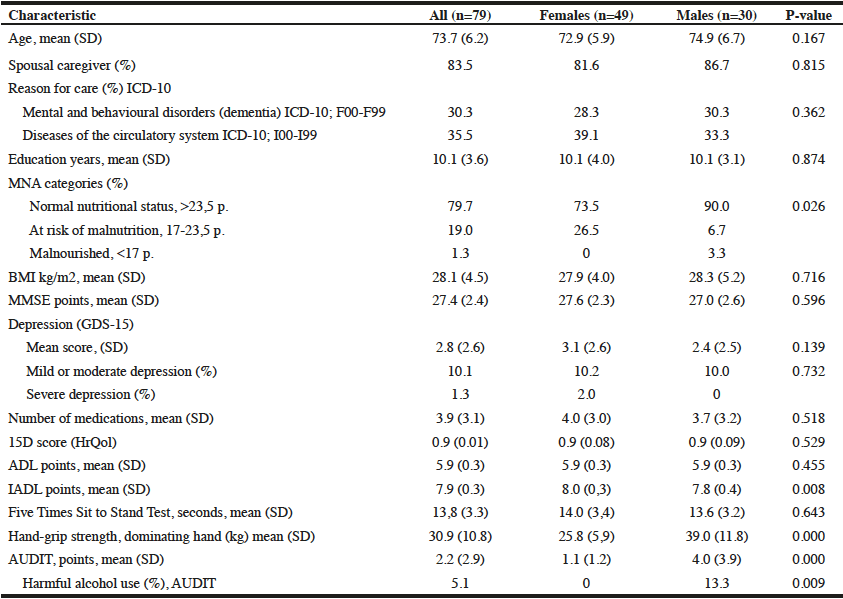
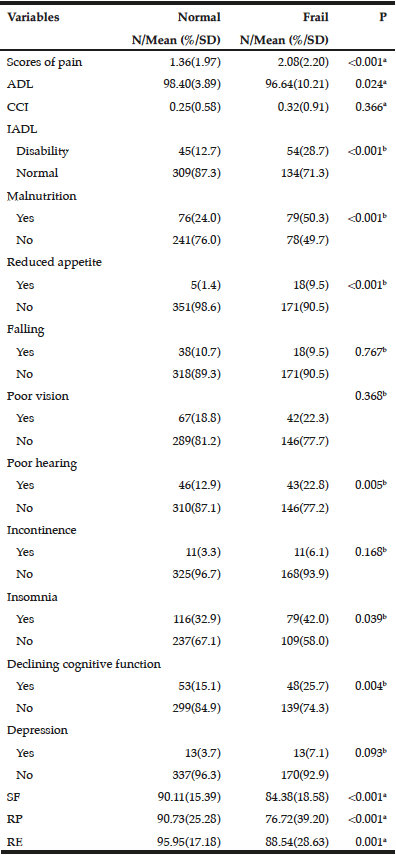
Records from 107 community dwelling older adults who came for care to the RSDM clinics in Newark, NJ between June 1, 2015 and July 15, 2016 were included in the analyses (Figure 1). Their mean age was 72.6 years (SD=5.6) with a range of 65-91 years. Table 1 describes demographic and clinical characteristics of participants.
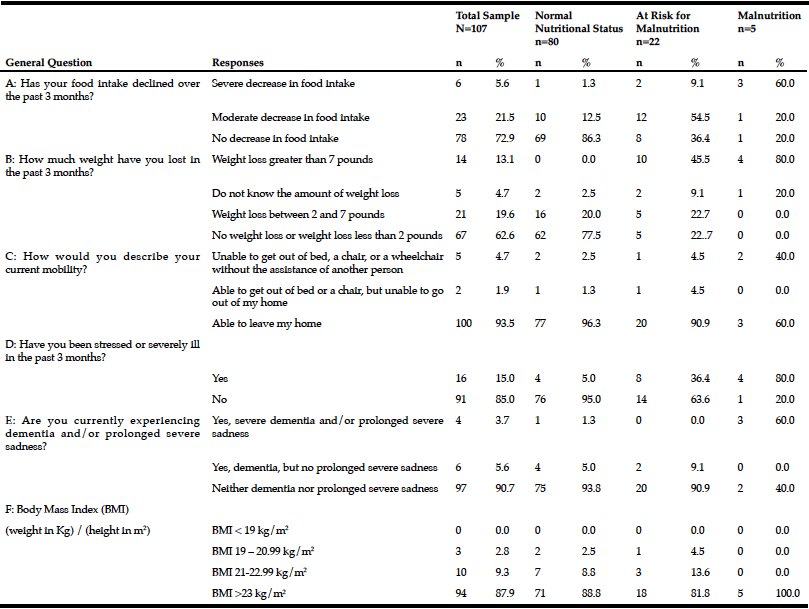
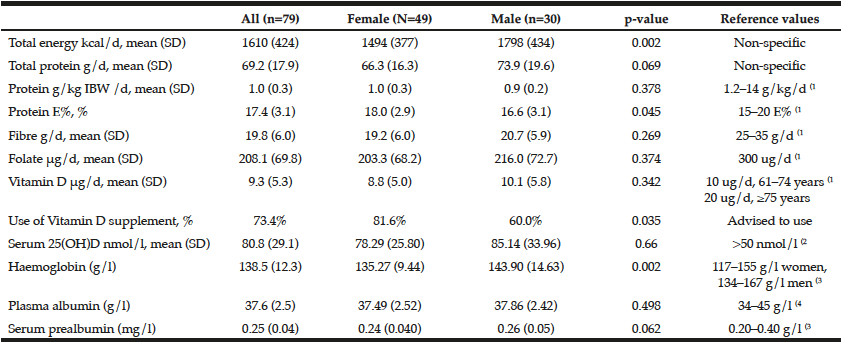
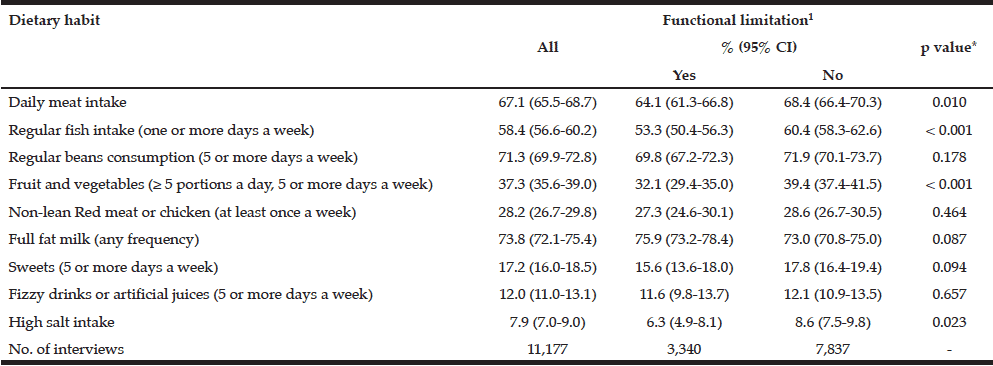
The mean Self-MNA score of this sample was 12.3 (SD=2.0) reflective of normal nutrition status. Twenty percent (20.6%, n=22) were at risk for malnutrition and 4.7% (n=5) were malnourished according to their Self-MNA responses. A moderate to severe decrease in food intake was reported by 27.1% (n=29) of the patients; 32.7% (n=35) indicated a weight loss of two or more pounds in the past three months (Table 2). Of those who reported a weight loss of more than seven pounds in three months (n=14), all were either at risk for malnutrition (n=10) or malnourished (n=4).
Of the five who were categorized as having malnutrition; 80% (n=4) reported a severe or moderate decrease in food intake, a weight loss greater than seven pounds in three months, and that they had been stressed or severely ill in the past three months; 60% (n=3) of these also had positive responses for severe dementia and/or prolonged sadness; 40% (n=2) were unable to get out of bed or a chair without assistance.
Using the Centers of Disease Control and Prevention (34) (CDC) BMI categories, 21.5% (n=23) were in the normal weight range, 43.0% (n=46) were overweight and 35.5% (n=38) were obese. All those whose Self-MNA score reflected malnutrition (n=5) were obese (mean BMI = 31.6, SD =4.5) with higher BMI values than those who had a normal nutritional status (mean BMI = 29.0, SD=5.0) or who were at risk for malnutrition (mean BMI = 27.6, SD=4.7).
Note: Sample size for each question varied as participants were able to choose to not answer questions on the registration forms.
The majority of participants were partially edentulous (n=94, 87.8%); 4.7% (n=5) were completely edentulous and 7.5% (n=8) were fully dentate. Fifty-one (47.7%) had at least 20 teeth, 29.9% (n=32) had 10-19 teeth and 22.4% (n=24) had 0-9 teeth. Approximately one-third (35.5%, n=38) of the sample had no posterior occlusion and 7.5% (n=8) had complete posterior occlusion; approximately one quarter (26.2%, n=28) had no anterior occlusion and 43.0% (n=46) had complete anterior occlusion.
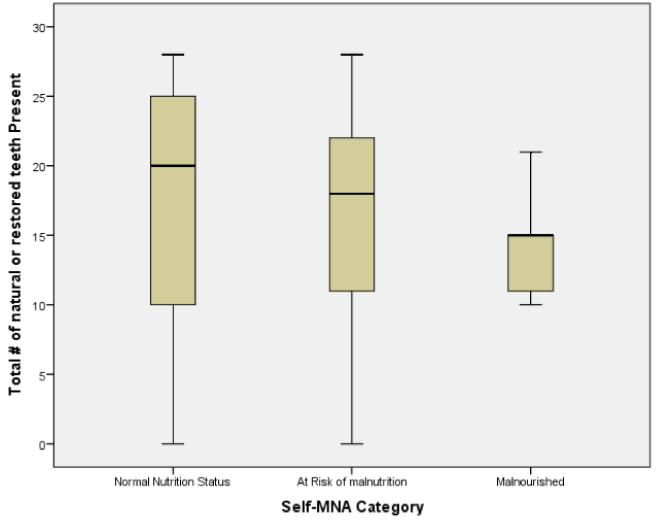
The mean number of natural or restored teeth decreased from 17.4 among those with normal nutritional status to 14.4 among those classified as having malnutrition; however, these differences were not statistically significant (p=0.656). Median values of teeth declined in a similar pattern, from 20 among those with normal nutritional status to 18 among those at risk for malnutrition and 15 among those categorized as having malnutrition (Figure 2).
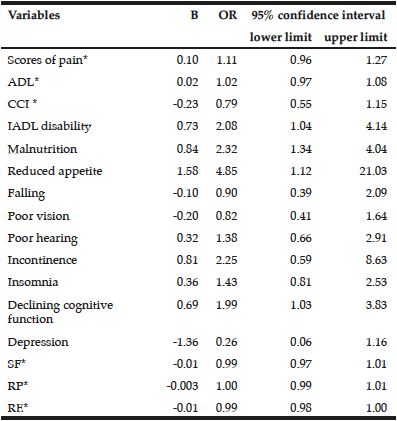
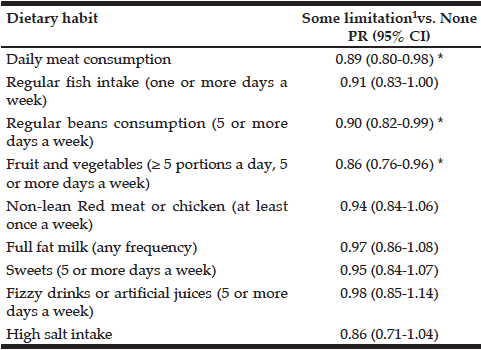
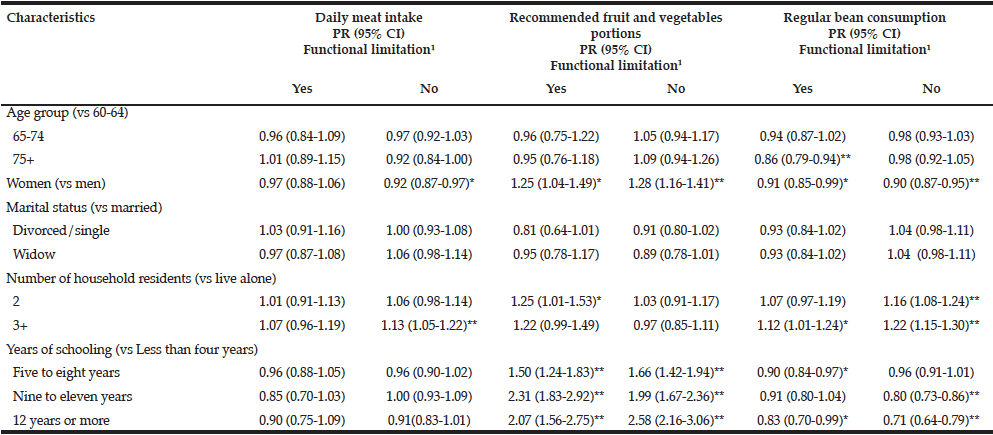
Analyses of the relationships between dental and occlusal status and Self-MNA Score revealed no linear relationships between the Self-MNA score and the number of natural teeth (r=0.104, p=0.285), posterior occluding pairs (POP) of teeth (r=0.173, p=0.074) or anterior occluding pairs (AOP) of teeth (r=0.049, p=0.619). Although mean differences were not significant (Kruskal-Wallis, p=0.116), those with 10-19 teeth had lower Self-MNA scores (mean=11.6, SD=2.5) than those with 0-9 teeth (mean=12.7, SD=1.3) or 20 or more teeth (mean=12.6, SD=1.8) (Figure 3). When MNA Score was dichotomized into those who were at risk for malnutrition or malnourished (25.2%, n=27) compared to those with normal nutritional status (74.8%, n=80), those with 10-19 teeth had 2.5 times the odds of being at risk for malnutrition/malnourished than those with 20 or more teeth (OR=2.5, p=0.076). Bivariate analyses yielded no statistically significant confounding effects of age, gender, race/ethnicity or medical history.
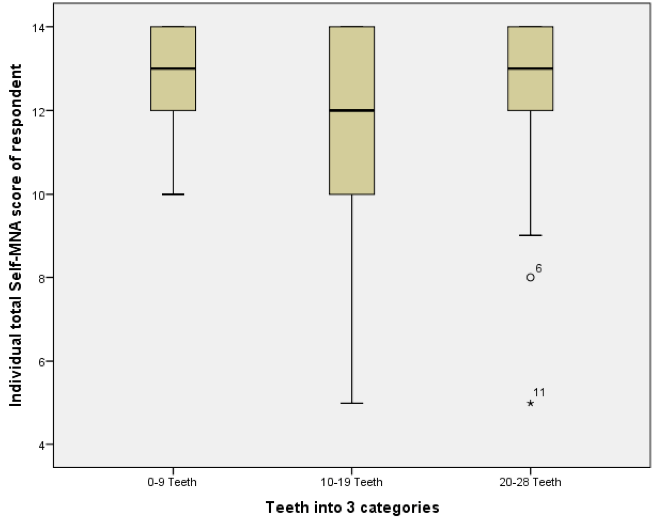
Figure 3
Boxplot of Self-MNA scores among those with different categories of natural or restored teeth (N=107)
Discussion
The aim of this study was to explore the associations between nutritional status and dentition status among older adults who presented for care at the RSDM clinics in Newark, NJ. The study hypothesis that Self-MNA scores will be significantly lower in those with fewer teeth / limited occlusion as compared to those with more teeth and/or more posterior occluding teeth pairs was not supported as a significant direct relationship between Self-MNA score and number of natural or restored teeth was not found.
The majority of the sample had some degree of tooth loss (87.8% partially edentulous; 4.7% completely edentulous). Although not statistically significant, there was a trend whereby the mean number of natural or restored teeth decreased from 17.4 in those with normal nutritional status to 14.4 in those classified as having malnutrition, suggesting a link between having fewer teeth and an increased risk of malnutrition. Those with 10-19 teeth had lower Self-MNA scores than those with 0-9 teeth or 20 or more teeth and, among those with 10-19 teeth the odds of being at risk for malnutrition/malnourished were 2.5 times those with 20 or more teeth. Interestingly, the patients with malnutrition had 10-19 teeth; none were fully dentate or fully edentulous. Furuta et al (17) also noted a similar trend whereby those with 10-19 teeth had the lowest MNA-SF scores as compared to those with 0-9 teeth or greater than 20 teeth. While not significant, this finding suggests that those who are missing one third to two thirds of their natural teeth may be most at risk for becoming malnourished.
In contrast to our findings, Kikutani et al (16), Starr et al (18), and Furuta et al (17), reported significant associations between MNA scores and number of missing teeth whereby MNA scores were significantly lower in those with fewer teeth / limited occlusion as compared to those with more teeth and/or more posterior occluding teeth pairs of teeth. Kikutani et al found that individuals with more missing teeth and inadequate occlusion were more likely to be at risk for malnutrition, and those with functionally inadequate occlusion and no dentures had a 3.189 fold greater malnutrition risk than those with natural dentition and adequate function (16). Similarly, Starr et al demonstrated that individuals who were completely edentulous had significantly lower MNA scores than those who were partially or completely dentate (p = 0.028) (18). In bivariate models, Furuta et al found that those with 0-19 teeth had lower MNA scores than those with 20 or more teeth (p = 0.041), however, in a multivariate path analysis, no direct relationship was noted between oral health status and MNA score (17).
While the results of the current study showed similar trends they did not reach the level of statistical significance reported by others. Possible explanations for the variation in results lie in the differences in the populations studied. The subjects in our study were all patients of an urban northeast US dental school clinic in Newark, NJ, and were younger than participants in other studies (16-18). The combined 25% prevalence of malnutrition and risk for malnutrition was relatively lower than the 13.3% malnourished and additional 51.7% at risk for malnutrition reported by Kikutani et al (16), and 14.0% malnourished / 55.2% at risk of malnutrition reported by Furuta et al (17). Participants in the current study had more teeth (mean of 17.0 ±8.5 compared to 8.6 +/- 9.9) (17) and were less likely to be edentulous then those studied by Furuta et al (17). In contrast, the population studied by Starr et al represented healthy older adults who were free of medical conditions and medications at baseline, and as a whole had relatively high MNA scores with a mean of 8.0 or greater out of a maximum score of 9 using their version of the MNA tool (18).
Our findings are consistent with preceding studies, which were unable to find statistically significant relationships between number of teeth (35), occlusal status (21) or dental status (22) and MNA score. Systematic reviews of the evidence (15, 23), have similarly found much conflicting results due in part to the heterogeneity of the studies comparing these variables. Nutritional status and dentition status can be assessed in a variety of ways. Varying clinical and demographic characteristics of the populations sampled such as age, health status and country of origin add to the complexity of this area of research.
Natural dentition and adequate function are important to maintain nutritional status. Missing teeth and inadequate occlusion affect masticatory function and can result in a decline in overall quality and quantity of intake (5-9) and/or replacement of difficult to chew foods with softer foods that may be more calorically dense and/or nutrient poor which could lead to changes in weight and overall nutritional status (36, 37). Further studies that address diet quality and quantity are necessary to better understand these relationships and guide implications for practice.
Strengths and Limitations
Although the study sample was adequately powered and representative of the population of older adults who attend the RSDM clinic, the majority were overweight/obese and of normal nutrition status. The findings are limited to a single institution and not generalizable to other community dwelling dental settings. Given the retrospective design of this study, a cause and effect relationship between variables could not be established. Risk for malnutrition may be better assessed using a tool such as the MNA-SF, which considers oral health factors specifically when looking at inadequate intake.
Strengths include data collection over a 13-month timespan to assure an adequate sample size, the availability of all of the data in the EHR, and the verification of dentition status by a research assistant, who was trained and calibrated with a prosthodontist, using digital radiography and the patient’s odontogram. The subjective nature of all self-reported data, including all demographic characteristics, height, weight, and Self-MNA data is a potential limitation. Although the Self-MNA is a validated tool to assess malnutrition prevalence and risk, it lacks the details found in the MNA-SF that prompts the healthcare professionals to assess the etiology behind a decline in food intake such as loss of appetite, digestive problems, chewing or swallowing difficulties (4).
Conclusion
The findings of this study did not support relationships between the number of natural or restored teeth and Self-MNA score in this sample of community dwelling older adults. Although not statistically significant, the mean number of natural or restored teeth declined as nutritional status declined, which may be clinically relevant. Those classified as having malnutrition had higher rates of weight loss, decreased intake and more frequently reported dementia and/or depression, and severe illnesses than those with a normal nutritional status. Similarly, those classified as being at risk of malnutrition were more likely to experience weight loss and a moderate decline in intake than those who were classified as being of normal nutritional status.
Implications for Research
Our findings add to the heterogeneity of research outcomes in this area. The conflicting findings between studies is in part due to the use of different measures of nutrition and dental status as well as variations in demographic characteristics. Further research with larger samples, in different populations of community dwelling older adults with varying health conditions is needed to better understand the associations between nutrition and oral health and increase the generalizability of results. Prospective studies to determine and compare changes in nutritional status over time with changes in dentition and denture usage can help to determine cause and effect. Qualitative research aimed at understanding how impaired dentition affects intake, the eating experience and overall nutritional status can also help to shed light on these relationships, and can provide the necessary data upon which to develop practice based interventions and guidelines for this vulnerable population. Although the Self-MNA has been validated as a self-administered tool to determine malnutrition prevalence and risk in community dwelling older adults, research in this area may benefit from using the MNA-SF, which is administered by a healthcare professional as older adults may require more clarification of questions to elicit more accurate results. The MNA-SF also includes a question related to decline in intake attributed to chewing or swallowing difficulties.
Implications for Practice
The dental clinic setting may be an ideal location to perform nutritional status screenings. As reported by Greenberg et al, both oral healthcare professionals and patients were receptive to screening for various medical conditions at the time of a dental visit (38, 39). This could be expanded to include nutritional status screening to identify patients at risk for malnutrition who may not regularly attend visits with a primary care provider. The results of the current study revealed that over 25% of the sample of older adults in this research who came for care at the RSDM clinic had malnutrition or were at risk for malnutrition. Based on these results and previous studies that estimated the prevalence of malnutrition to be over one third in older adults (3, 4), the use of nutritional screening tools by oral healthcare professionals could help to provide timely referrals to primary care physicians or Registered Dietitian Nutritionists for these individuals. Referrals to community assistance programs (such as Meals on Wheels) could also be made as appropriate to prevent decline in nutrition status.
Ethical standards: This study was approved by the Rutgers University Biomedical and Health Sciences Institutional Review Board.
Funding agency: Sackler Institute for Nutritional Sciences. PI Rena Zelig received an early career investigator’s grant from the Sackler Institute for Nutrition Sciences for a project entitled “Exploring the Associations between Dentition Status, Nutritional Status, and the Eating Experience in Older Adults – A Mixed Methods Study.” The sponsors had no role in the design and conduct of the study; in the collection, analysis, and interpretation of data; in the preparation of the manuscript; or in the review or approval of the manuscript.
Acknowledgements: Funding for this project was provided by the Sackler Institute for Nutritional Sciences of the New York Academy of Sciences. The authors wish to acknowledge Dr. Michael Conte and the Office of Clinical Affairs and the Information Technology Department at the Rutgers School of Dental Medicine, Newark, NJ as well as Steven Britton, DMD, and Veronica Jones, MPH, Research Assistants for their help with this study.
Conflict of Interest Disclosure: All authors report that they have no conflicts of interest to disclose related to this research and manuscript.
References
1. Petersen PE, Kandelman D, Arpin S, Ogawa H. Global oral health of older people–call for public health action. Community Dent Health 2010;27(4 Suppl 2):257-67
2. Dye BA, Thornton-Evans G, Li X, Iafolla TJ. Dental Caries and Tooth Loss in Adults in the United States, 2011–2012. NCHS data brief, no 197. Hyattsville, MD. National Center for Health Statistics. 2015. Available at:
https://www.cdc.gov/nchs/data/databriefs/db197.pdf. Accessed January 12, 2018.
3. Kaiser MJ, Bauer JM, Ramsch C, et al. Frequency of malnutrition in older adults: a multinational perspective using the mini nutritional assessment. J Am Geriatr Soc 2010;58(9):1734-8 doi: 10.1111/j.1532-5415.2010.03016.
4. Huhmann MB, Perez V, Alexander DD, Thomas DR. A self-completed nutrition screening tool for community-dwelling older adults with high reliability: a comparison study. J Nutr Health Aging 2013;17(4):339-44 doi: 10.1007/s12603-013-0015-x
5. Sahyoun NR, Lin CL, Krall E. Nutritional status of the older adult is associated with dentition status. J Am Diet Assoc 2003;103(1):61-6 doi: 10.1053/jada.2003.50003
6. Sheiham A, Steele J. Does the condition of the mouth and teeth affect the ability to eat certain foods, nutrient and dietary intake and nutritional status amongst older people? Public Health Nutr 2001;4(3):797-803
7. Zhu Y, Hollis JH. Tooth loss and its association with dietary intake and diet quality in American adults. J Dent 2014;42(11):1428-35 doi: 10.1016/j.jdent.2014.08.012
8. Iwasaki M, Taylor GW, Manz MC, et al. Oral health status: relationship to nutrient and food intake among 80-year-old Japanese adults. Community Dent Oral Epidemiol 2014;42(5):441-50
9. Ervin RB, Dye BA. Number of natural and prosthetic teeth impact nutrient intakes of older adults in the United States. Gerodontology 2012;29(2):e693-702 doi: 10.1111/j.1741-2358.2011.00546.x
10. Zajacova A, Ailshire J. Body Mass Trajectories and Mortality Among Older Adults: A Joint Growth Mixture-Discrete-Time Survival Analysis. Gerontologist 2013 doi: 10.1093/geront/gns164
11. Lundin H, Saaf M, Strender LE, Mollasaraie HA, Salminen H. Mini nutritional assessment and 10-year mortality in free-living elderly women: a prospective cohort study with 10-year follow-up. Eur J Clin Nutr 2012;66(9):1050-3 doi: 10.1038/ejcn.2012.100
12. Lorenzo-Lopez L, Maseda A, de Labra C, Regueiro-Folgueira L, Rodriguez-Villamil JL, Millan-Calenti JC. Nutritional determinants of frailty in older adults: A systematic review. BMC Geriatr 2017;17(1):108 doi: 10.1186/s12877-017-0496-2
13. Favaro-Moreira NC, Krausch-Hofmann S, Matthys C, et al. Risk Factors for Malnutrition in Older Adults: A Systematic Review of the Literature Based on Longitudinal Data. Adv Nutr 2016;7(3):507-22 doi: 10.3945/an.115.011254
14. van der Pols-Vijlbrief R, Wijnhoven HA, Schaap LA, Terwee CB, Visser M. Determinants of protein-energy malnutrition in community-dwelling older adults: a systematic review of observational studies. Ageing Res Rev 2014;18:112-31 doi: 10.1016/j.arr.2014.09.001
15. Zelig R, Touger-Decker R, Chung M, Byham-Gray L. Associations between Tooth Loss, with or without Dental Prostheses, and Malnutrition Risk in Older Adults: A Systematic Review. Top Clin Nutr 2016;31(3):232-47
16. Kikutani T, Yoshida M, Enoki H, et al. Relationship between nutrition status and dental occlusion in community-dwelling frail elderly people. Geriatr Gerontol Intl 2013;13(1):50-4 doi: 10.1111/j.1447-0594.2012.00855.x
17. Furuta M, Komiya-Nonaka M, Akifusa S, et al. Interrelationship of oral health status, swallowing function, nutritional status, and cognitive ability with activities of daily living in Japanese elderly people receiving home care services due to physical disabilities. Community Dent Oral Epidemiol 2013;41(2):173-81 doi: 10.1111/cdoe.12000
18. Starr JMH, R. J.;Macintyre, S.;Deary, I. J.;Whalley, L. J. Predictors and correlates of edentulism in the healthy old people in Edinburgh (HOPE) study. Gerodontology 2008;25(4):199-204
19. Cousson PY, Bessadet M, Nicolas E, Veyrune JL, Lesourd B, Lassauzay C. Nutritional status, dietary intake and oral quality of life in elderly complete denture wearers. Gerodontology 2012;29(2):e685-92 doi: 10.1111/j.1741-2358.2011.00545.x
20. McKenna G, Allen PF, Flynn A, et al. Impact of tooth replacement strategies on the nutritional status of partially-dentate elders. Gerodontology 2012;29(2):e883-90 doi: 10.1111/j.1741-2358.2011.00579.x
21. Soini H, Routasalo P, Lauri S, Ainamo A. Oral and nutritional status in frail elderly. Spec Care Dent 2003;23(6):209-15
22. Soini H, Routasalo P, Lagstrom H. Nutritional status in cognitively intact older people receiving home care services–a pilot study. J Nutr Health Aging 2005;9(4):249-53
23. Toniazzo MP, Amorim PS, Muniz FW, Weidlich P. Relationship of nutritional status and oral health in elderly: Systematic review with meta-analysis. Clin Nutr 2017 doi: 10.1016/j.clnu.2017.03.014
24. Dye BA, Li X, Thornton-Evans G. Oral Health Disparities as Determined by Selected Healthy People 2020 Oral Health Objectives for the United States, 2009–2010. NCHS data brief: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics, 2012.
25. National Institutes of Health. National Institute of Dental and Craniofacial Research. Tooth Loss in Seniors (Age 65 and Over). Tooth Loss in Seniors (Age 65 and Over) March 11, 2014. Available at: https://www.nidcr.nih.gov/DataStatistics/FindDataByTopic/ToothLoss/ToothLossSeniors65andOlder.htm. Accessed January 12, 2018.
26. Bureau USC. QuickFacts. Newark city, New Jersey. Available at: https://www.census.gov/quickfacts/table/PST045215/3451000,34. Accessed January 12, 2018.
27. Guigoz Y, Vellas B. The Mini Nutritional Assessment (MNA) for grading the nutritional state of elderly patients: presentation of the MNA, history and validation. Nestle Nutrition workshop series. Nestle Nutr Workshop Ser Clin Perform Programme 1999;1:3-11; discussion 11-2.
28. Rubenstein LZ, Harker JO, Salva A, Guigoz Y, Vellas B. Screening for undernutrition in geriatric practice: developing the short-form mini-nutritional assessment (MNA-SF). J Gerontol A Biol Sci Med Sci 2001;56(6):M366-72
29. Kaiser MJ, Bauer JM, Ramsch C, et al. Validation of the Mini Nutritional Assessment short-form (MNA-SF): a practical tool for identification of nutritional status. J Nutr Health Aging 2009;13(9):782-8
30. Yoshida M, Kikutani T, Yoshikawa M, Tsuga K, Kimura M, Akagawa Y. Correlation between dental and nutritional status in community-dwelling elderly Japanese. Geriatr Gerontol Intl 2011;11(3):315-9 doi: 10.1111/j.1447-0594.2010.00688.x
31. Ostberg AL, Nyholm M, Gullberg B, Rastam L, Lindblad U. Tooth loss and obesity in a defined Swedish population. Scand J Public Health 2009;37(4):427-33
32. Syrjala AMY, P.;Hartikainen, S.;Sulkava, R.;Knuuttila, M. Number of teeth and selected cardiovascular risk factors among elderly people. Gerodontology 2010;27(3):189-92
33. Portney L, Watkins M. Foundations of Clinical Research, Applications to Practice. 3 ed. Upper Saddle River, NJ: Pearson Education, Inc, 2009.
34. Centers for Disease Control and Prevention. Healthy Weight. Secondary Healthy Weight. Available at: http://www.cdc.gov/healthyweight/assessing/bmi/adult_bmi/index.html. Accessed January 12, 2018.
35. Lopez-Jornet P, Saura-Perez M, Llevat-Espinosa N. Effect of oral health dental state and risk of malnutrition in elderly people. Geriatr Gerontol Int 2013;13(1):43-49 doi: 10.1111/j.1447-0594.2012.00853.x
36. Musacchio EP, E.;Binotto, P.;Sartori, L.;Silva-Netto, F.;Zambon, S.;Manzato, E.;Corti, M. C.;Baggio, G.;Crepaldi, G. Tooth loss in the elderly and its association with nutritional status, socio-economic and lifestyle factors. Acta Odontol Scand 2007;65(2):78-86
37. Ikebe KM, K.;Morii, K.;Nokubi, T.;Ettinger, R. L. The relationship between oral function and body mass index among independently living older Japanese people. Int J Prosthodont 2006;19(6):539-46
38. Greenberg BL, Glick M, Frantsve-Hawley J, Kantor ML. Dentists’ attitudes toward chairside screening for medical conditions. J Am Dent Assoc 2010;141(1):52-62
39. Greenberg BL, Kantor ML, Jiang SS, Glick M. Patients’ attitudes toward screening for medical conditions in a dental setting. J Public Health Dent 2012;72(1):28-35 doi: 10.1111/j.1752-7325.2011.00280.x