J.F. Abrahamsen1, R. Rozzini2, S. Boffelli2, A. Cassinadri2, A.H. Ranhoff3, M. Trabucchi4
1. Departement of Nursing Home Medicine, Municipality of Bergen and Kavli Research Centre for Geriatrics and Dementia, Haraldsplass Deaconess Hospital, Bergen, Norway; 2. Fondazione Ospedale Poliambulanza, Brescia and Geriatric Research Group, Brescia, Italy; 3. Kavli Research Centre for Geriatrics and Dementia, Haraldsplass Deaconess Hospital, Bergen, and Departement of Clinical Science, University of Bergen, Norway; 4. Professor of Neuropsycopharmacology, University of Rome II, and Geriatric Research Group, Brescia, Italy
Corresponding Author: Jenny Foss Abrahamsen, Departement of Nursing Home Medicine, Municipality of Bergen and Kavli Research Centre for Geriatrics and Dementia, Haraldsplass Deaconess Hospital, Ulriksdal 8, 5009, Bergen, Norway. jennyfossabrahamsen@gmail.com Telephone: 004799514977
Abstract
Background/Objectives: Little is known regarding the influence of sociodemographic and clinical factors, on the short term outcomes of different postacute care (PAC) models in different countries. Design and setting: Prospective cohort study of a 19- bed Italian hospital subacute care (SAC) unit and a 19-bed Norwegian nursing home (NH) intermediate care (IC) unit, both based on Comprehensive Geriatric Assessment and similar multidisciplinary staffing. Participants: A total of 664 Italian and 961 Norwegian community-dwelling patients ≥70 years of age, in need of postacute geriatric based treatment, rehabilitation and care. The patients were admitted from acute medical, surgical and orthopaedic hospital units. Measurements: Demographic data, clinical information, comprehensive geriatric assessment (CGA), discharge destination and length of stay were recorded in an Italian and a Norwegian database and compared. Results: The Italian patients receiving hospital SAC, were more seriously affected by the acute disease and trauma, median Barthel index (BI) at admission/discharge was 40/60, compared to 75/85 in the Norwegian patients, and fewer of them were able to return to own home as compared to the Norwegian patients ( 64% vs. 82%). Although the Italian patients had a lower BI at discharge, fewer of them were transferred to nursing homes (9%), as compared to the Norwegian patients (14%), while more of them were discharged to further rehabilitation, acute hospitalization, hospice or died (27%), as compared to the Norwegian patients (4%). Of the patients discharged to own home, only 8% of the Italian compared to 71% of the Norwegian patients received nurse assisted home care. Admission BI and improvement in BI, were highly significant predictors for the ability to return home in multivariate logistic regression analysis both in the Italian and the Norwegian patients. Conclusions: Both clinical and sociodemographic factors influenced the clinical outcome of older patients receiving PAC in Italy and Norway. Such differences should be taken into account when results from different PAC models in different countries are compared. Both the Italian hospital SAC model and the Norwegian NH IC model are feasible and good alternatives, but more firm inclusion criteria may further optimize the selection of patients suitable for different PAC options.
Key words: Postacute care, subacute care, intermediate care, nursing home, older patients.
Introduction
Hospitalization for acute disease or injury may, in older home- dwelling patients, be associated with functional decline and increasing dependency (1-3). Some patients are not able to return to their own home after acute hospitalization and need further multidimensional geriatric based care to regain their functional capacity.
There are numerous facilities that offer this kind of care, different terms are used, different patients are selected and different kind of care is offered.
In the present article we define Post-Acute Care (PAC) as different treatment modalities available after an acute hospital stay to further the goals of acute care (4, 5). PAC facilities may be located in hospitals, rehabilitation centers or nursing homes. Older adults who have overcome the acute phase of their hospitalization but are not able to carry out functional tasks with the level of independence required to return to their previous accommodation, may be considered candidates for PAC.
In England and other European countries, the term Intermediate care (IC) has been used as synonymous/overlapping with PAC (6-8). In the present study we define IC as one type of PAC offered to older patients after acute hospitalization with the main intention to provide active treatment and rehabilitation in the community after hospital discharge. IC services are generally, but not always, based on community-based interdisciplinary teams.
Another form of PAC is SubAcute Care (SAC), which focuses on inpatient multidisciplinary geriatric treatment and rehabilitation (9, 10). This care model is a complement to acute and curative medicine and share the aims of other PAC models.
While earlier studies have demonstrated beneficial effects of both community based IC (11) and hospital based SAC (12), there is an ongoing discussion regarding the content of, and which patients are best suited for different models (8, 13, 14). Furthermore, there is a lack of evidence comparing different PAC models and rehabilitation results between healthcare settings from different Europeans countries.
Since geriatric research collaboration earlier had been taken place between Italy and Norway and both countries recently had established new PAC treatments modalities, a new study was set up with the goal to describe and compare these two different PAC models. The Italian model was an inpatient hospital SAC model, while the Norwegian model was a skilled nursing home (NH) IC model. However both models were based on Comprehensive Geriatric Assessment (CGA) and had comparable multidisciplinary staffing.
The overall aim of the present study was to describe and assess the feasibility and benefits the two different PAC models, and to investigate how sociodemographic, clinical and health care features affected the short term treatment results.
Methods
Overall aim of the two postacute care models
The overall aim of both models was to relieve pressure on the acute hospital beds and to deliver CGA based treatment and rehabilitation for older patients that did not need to stay in an acute hospital unit, but still had more complex medical and functional needs than could be managed at home. The final goal was that the patients should be able to return to their own home.
The 19-bed Italian SAC unit was established in 2011 as part of the geriatric department at the Fondazione Ospedale Poliambulanza in Brescia, Italy (15). In addition to treating and rehabilitate patients after an acute hospital admission, this treatment option was also available for home dwelling elderly patients with chronic disease to avoid early flare-up, relapse and acute hospitalization.
The 19-bed Norwegian IC unit was established in 2005 as a collaboration between the municipality of Bergen, and the two hospitals serving the town (16-18). Emphasis was put on selecting patients from the acute medical and orthopaedic hospital departments that had a treatment and rehabilitation potential, and that the treatment period should be rather short, preferably ≤14 days, to allow a rather high turnover of patients that were able to receive CGA based treatment and care.
Patient selection
The inclusion criteria for the Italian and Norwegian units are shown in Table 1.
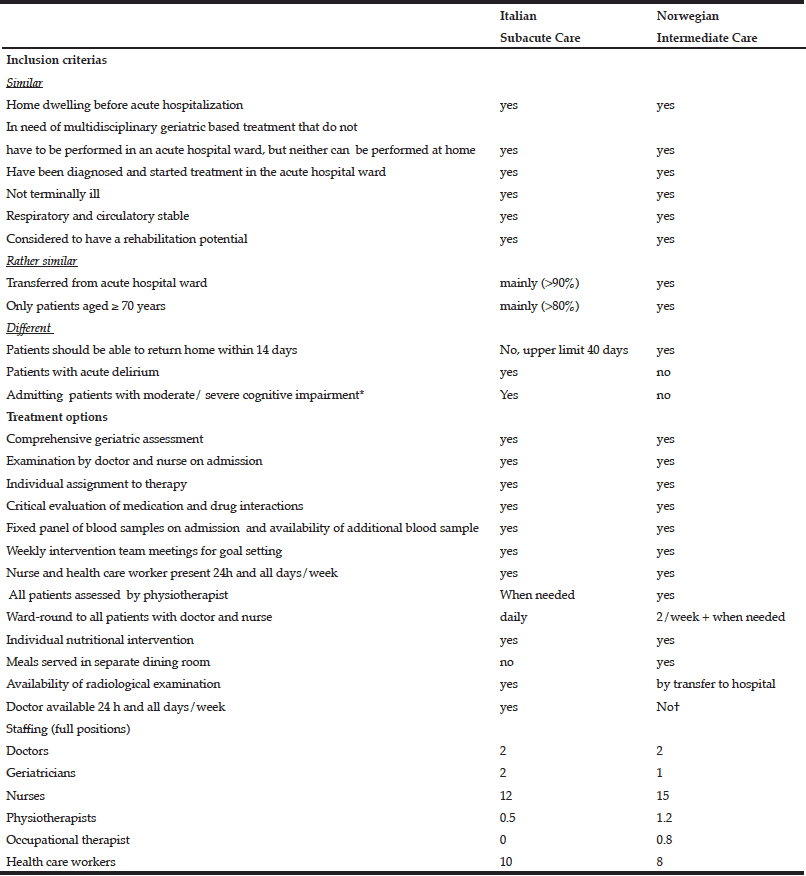
Table 1 Inclusion criteria and treatment options for older patients receiving hospital subacute care (SAC) in Italy and nursing home intermediate care (IC) in Norway
Abbreviations: IADL-instrumental activities of daily life, CIRS, Cumulative Illness Rating Scale comorbidity and severity, CDR Clinical Dementia Rating, MNA-SF, TUG- Timed up and go, Mini Nutritional Assessment-Short Form; *As long as the patient has medical needs and the cognitive decay was not the reason for the admittance. †Nursing home doctor only present at ordinary daily working hours and not in weekends. Nursing home doctor on call for all the nursing homes in Bergen could be contacted and visit the patient. Otherwise, at nights, if needed, the nursing home nurse could call the hospital directly for advise or readmittance of the patients
In the Italian SAC unit, the doctor in the acute hospital ward or less frequently, the patient`s family physician (if the patient were not yet admitted to hospital) would call the geriatrician in the SAC unit that would decide whether the patient was suitable for admittance. From 2014 and onwards, major cognitive impairment was no exclusion criteria, as long as the patient had medical needs and the cognitive decline was not the reason for the admittance. The Italian SAC unit also admitted patients with acute delirium. Patients in immediate needs of nursing home were excluded.
In the Norwegian IC unit the selection process was as follows: 1)The hospital doctor selected patients that needed further medical treatment and rehabilitation, according to the inclusion criteria. 2) The hospital doctor or nurse phoned the NH giving a short report on the patient including, diagnosis, social status, physical ability and purpose of admission to intermediate care. 3) The NH doctor decided, based on the information given from the hospital, whether the patient was suitable for IC. Approximately 80 % of the referred patients were considered suitable for transferral from the hospital to the IC unit.
Design, setting and treatment options
The treatment options given by the Italian SAC unit and the Norwegian IC unit are shown in Table 1. In both units, in addition to continuation of the medical treatment started in the acute hospital, multidisciplinary CGA-based treatment was given according to the patient`s needs, e.g. physiotherapy, nursing care and social and nutritional intervention. Treatment plans and decisions about discharge and making arrangements for further treatment and care after discharge, were conducted after discussion in the multidisciplinary team.
Both treatment modalities were based on care in an environment adjusted to elderly patients, with main emphasis on multidisciplinary staffing, ADL training, nutrition and social wellbeing. Furthermore, communication with the family, community nurse- and physiotherapy service, to improve the patients home condition was essential to enable a safe return to their own home and avoid further NH transferral.
The patients in the Italian SAC unit had all the acute hospital facilities available, including the possibility of 24-hour doctor visits, blood samples, radiological investigations and rapid transfer to the acute departments. However, main emphasis was put on avoiding unnecessary further investigations and rather offering multidisciplinary treatment to achieve maximum independence. Extensive geriatric assessment was performed with Barthel index (BI)(19), Mini Mental Status Examination (MMSE) (20), 15 item Geriatric Depression scale (GDS) (21) and Tinetti scale (22) by the doctor when admitting the patients and the day before discharge, Cumulative Illness Rating Scale (CIRS) severity and comorbidity (23), Blaylock scale (24), and Instrumental ADL (I-ADL) (25) at admission. In addition, the patient or their relatives were asked for information on the status of the patient 14 days before the hospital admission concerning BI, I-ADL and Clinical Dementia Rating (CDR) (26).
Information of the patients’ home situation and caregivers were obtained at admission. In Italy, the family usually makes high effort to take care of the patients after return to own home. Otherwise the patient or his/her family may hire a personal assistant living with the patient, or the hospital would ask the municipality for nurse assisted home care for personal needs. If the patient needed further rehabilitation, they were discharge to a geriatric rehabilitation centre. Patients who lacked a rehabilitation potential, but were unable to return home were transferred to a skilled nursing facility or hospice. Patients that deteriorated and became medically unstable with potential ability to reverse were readmitted to acute hospital units.
The Norwegian IC NH patients were after a short stay in the hospital for establishing the diagnosis and start of therapy, transported to the NH that was located 3 km away. Main emphasis was made on mobilizing the patients out of bed and out of the room, and that the patient should transfer (possible with aid) to the dining room for all meals. All patients were assessed by a physiotherapist and sometimes an occupational therapist, offered individual physiotherapy and group-based exercise. CGA was performed on all of the patients during the first week. Activities of daily living (ADL) were assessed by nurses observing the patients with the BI at the day of admission and at the day before discharge. Within the first week, the Norwegian version of the MMSE (20, 27), 30-item GDS (21), and Mini Nutritional Assessment- Short Form (MNA-SF) (28) was performed. The IC unit was supplied with equipment for intravenous treatment, blood transfusions, nebulizer for inhalation, bladder scan, 24-hour blood pressure recording, pulse oximetry and oxygen supply. Some blood tests could be analysed on the spot (haemoglobin, C-reactive protein and glucose), other blood samples were sent to the hospital laboratory for analysis within a few hours, when needed. NH doctors were only present at ordinary daily working hours and not in weekends. At other times a NH doctor on call for all the nursing homes in Bergen could be contacted and visit the patient or the NH nurse could, at night time, when needed, call the hospital directly for advice or readmission of the patients.
Nurse assisted home care and follow up by the physiotherapist in the community was offered to all patients that were in need of this, when they were discharged to their own home. If the patient could not return home within 14 days, transfer to an ordinary, lower-cost, skilled nursing facility should occur, however in a few instances exceptions were made. If the patients had a further rehabilitation potential they were further transferred to a NH with more physiotherapy resources. If the patients were not adequately medically diagnosed or stabilised, they were readmitted to hospital (acute hospitalization).
Data collection and Statistical analysis
The data on the patient`s demographic, baseline clinical characteristics and discharge destination, were obtained from hospital or IC nursing home records and included prospectively in an Italian and a Norwegian database, respectively. The data were analysed with standard descriptive statistics using the Statistical Package for Social Science IBM SPSS 20 for Windows. Continuous variables that had a normal distribution (age and days in PAC) was described as mean and SD otherwise the variables were described as median and min-max values.
For identifying characteristics that were independently associated with return to own home, odds ratios (ORs) with 95% confidence intervals (CIs) were estimated using logistic regression models. Variables that were available in both the Italian and Norwegian patients were included in univariate analysis (age, sex, BI admission, BI improvement (BI discharge – BI admission), MMSE and GDS). The variables associated with p < 0.25 in univariate analysis were noted as likely predictors and included in multivariate, adjusted logistic regression models. In this analysis, p ≤ 0.01 was considered to be statistically significant to account for multiple testing.
Ethics
The study was performed in appliance with the Helsinki Declaration(29), and no experimental interventions were performed.
In Italy, the study was approved by hospital Ethical board. Patients were informed about the study at admission. If the relative was absent, information was given at the first meeting with the family. Information was given mainly orally, while written consent was obtained for the treatment of personal data, and collected in the clinical charts. Consent for patients with severe cognitive impairment was obtained by the legal tutor. For other patients, consent was written, in presence of a relative. All of the consecutively admitted patients agreed to sign the informed consent.
In Norway the study was approved by the Regional Committee for Medical and Health Research Ethics. Information of the study was given by the doctor when admitting the patients, along with the written informed consent, that the patients would sign during the stay, possibly after consulting his/her family. No patients with major cognitive impairment or delirium was included.
Results
Patients` characteristics
During 2011-2014, 798 consecutive Italian patients were admitted to the SAC unit, 134 patients had age <70 years, thus altogether 664 Italian patients with age≥ 70 years were included in the present study. Of 1085 consecutive Norwegian patients with age ≥70 years and admitted to the IC unit, 112 were not asked to participate in the study at times when the geriatrician in charge was absent, 5 refused to participate, 4 had acute delirium and 2 had language problems. Thus 961 Norwegian patients were included.
As shown in Table 2, a majority of the Italian and Norwegian patients were admitted from the departments of internal medicine, cardiology, pulmonology, and in Italy, an acute geriatric department. Most of the medical patients had cardiovascular diseases or infections; many had started intravenous antibiotic therapy in the hospital. The Norwegian IC unit included more patients from the orthopaedic departments. Most of these patients had suffered a fall, including 76 hip fracture patients. None were admitted after elective surgery.
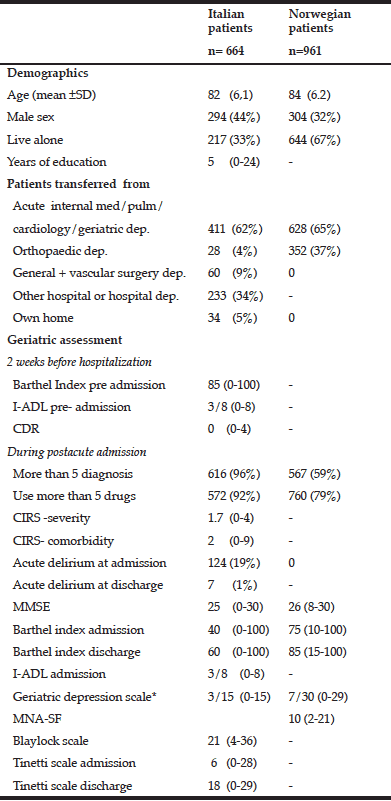
Table 2 Characteristics of Italian patients treated in a hospital subacute care unit and Norwegian patients treated in a nursing home intermediate care unit
Abbreviations: CIRS, Cumulative Illness Rating Scale, MMSE, Mini-Mental-Status Examination, I-ADL, Instrumental Activities of Daily Life, MNA-SF, Mini Nutritional Assessment- Short Form, CDR, Clinical Dementia Rating. Categorical variables are described as numbers and % of patients. Numerical variables, except age, are described as median and min-max.; *Italian patients assessed with 15 item scale, Norwegian population with 30 item scale
As shown in Table 2, fewer Italian patients were living alone, as compared to the Norwegian patients. Functional status 2 weeks before hospital admission indicated that the Italian patients, in general, were rather independent in ADL (BI= 85) and did not have major cognitive impairment (CDR=0), before the acute hospital admission. However more of them had >5 diagnoses and were using > 5 drugs, a higher percentage than the Norwegian patients. Furthermore, CGA indicated that the Italian patients in general were more severely affected by the acute disease, they had worse scores on all physical function and ADL assessment tests, and 19% of them had delirium on admission.
Outcome at discharge
The Italian patients experienced a substantial improvement in functional status, as shown in Table 3. However, as their admission ADL was low, their BI at discharge was still lower than that of the Norwegian patients, 60 versus 85, respectively (Table 2).
Despite that the Italian patients in general had a lower discharge BI, fewer of them were transferred to NH (9%), as compared to the Norwegian patients (14%). The Ital patients that were discharged to NH had lower median scores both on ADL assessment, BI 40(0-80) vs 60 (15-100) and MMSE 18 (0-30) vs 23 (8-30), as compared to the Norwegian patients.
Although the Norwegian patients that were discharge home had higher median BI score at discharge, 85 (35-100), compared to the Italian patients, 75 (5-100), more of them received nurse assisted home care after arriving home (Table 3). However, twice as many of the Norwegian patients were living alone (67% vs 33% (Table 3). As the majority of the Norwegian patients had a higher functional status and were able to return to their own home, fewer of them were transferred to further rehabilitation, acute hospitalization, hospice, or died during post-acute care, as compared to the Italian patients.
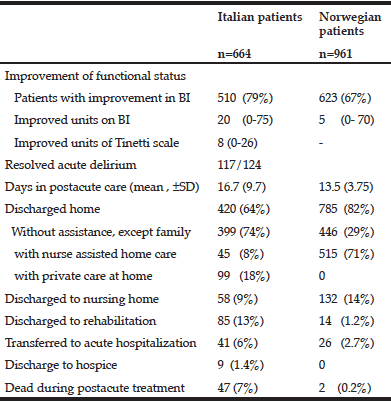
Table 3 Outcome at discharge in Italian patients treated in a hospital subacute care unit and Norwegian patients treated in a nursing home intermediate care unit
Abbreviations: CIRS, Cumulative Illness Rating Scale, MMSE, Mini-Mental-Status Examination; I-ADL, Instrumental Activities of Daily Life, MNA-SF, Mini Nutritional Assessment- Short Form, CDR, Clinical Dementia Rating. Categorical variables are described as numbers and % of patients
The Italian patients had a slightly longer stay, and fewer patients were discharged to own home. Possible predictors for the ability to return home was assessed by logistic regression analyses. Table 4 shows that in multivariate analysis, the admission BI and improvement in BI remained significant predictors for both the Italian and Norwegian patients.
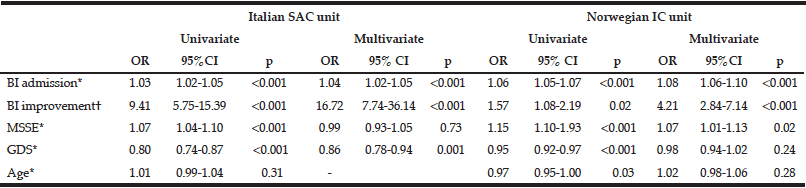
Table 4 Simple and multiple logistic regression for predictors of return to own home in Italian patients treated in a hospitalsubacute care unit and Norwegian patients treated in a nursing home intermediate care unit
SAC= Sub Acute Care, IC= Intermediate Care, OR= odds ratio, CI=confidence interval, BI, Barthel index, MMSE, Mini Mental State Examination, GDS, Geriatric; Depression Scale. OR were estimated using logistic regression models and adjusted for the covariates as described in the Methods section; *Variables are per unit increase, †Experienced any BI improvement during postacute care
Geriatric resources in Brescia, Italy and Bergen, Norway
Table 5 show the comparison of geriatric hospital care and nursing home care in the Municipalities of Brescia, Italy and Bergen, Norway.
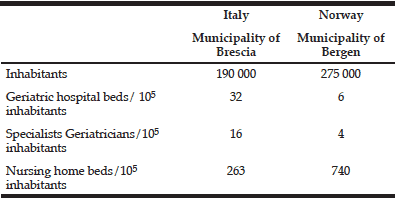
Table 5 Comparison of geriatric hospital care and nursing home care in the Municipalities of Brescia, Italy and Bergen, Norway
Discussion
The present study shows the influence of clinical and sociodemographic factors, regarding the short-term treatment and rehabilitation outcome of older patients cared for in an Italian hospital SAC unit and a Norwegian NH IC unit, both based on CGA and with comparable multidisciplinary staffing.
To our knowledge, no similar cross- national comparison has been done on different PAC settings, although a study comparing two hospital geriatric rehabilitation departments in Italy and Israel has previously been published (10). This study concluded that differences in sociodemographic and clinical factors could not account for all differences in ADL improvement that was observed between the two patients groups, and that the organization of care and constraints of the health system also influence functional outcome.
Regarding the rehabilitation outcomes after different PAC models, a Cochrane database review has concluded that there were insufficient evidence to compare the effects of NH, hospital and own home environments on older patients` rehabilitation outcomes (30), while a systematic review and meta-analysis of randomized controlled trials concludes that different hospital SAC models had the potential to improve outcomes relative to function, admission to NH and mortality (9). Intermediate care models are more heterogeneous (8, 31), but somewhat comparable facilities to the Norwegian NH IC unit have reported improved short-term functional recovery, improved long-term functional recovery and decreased mortality (5, 11).
In the present study, fewer Italian patients were discharged to NH and received home assisted nurse care, although their clinical condition was worse than that of the Norwegian patients. We believe that this probably is due to the influence of different sociodemographic and health care system factors: in Italy, more family care as compared to community based home nurse care, and a higher personal patient charge combined with less availability of nursing homes, as compared to Norway.
As indicated in Table 5, Italian geriatric hospital medicine is better developed with more hospital geriatric beds and more geriatricians, as compared to Norway, where a major political aim over the last years has been to treat elderly patients outside the hospital, in their local community, where the family physician is the primary health service provider. These differences may contribute to the set-up of an in-hospital SAC unit in Italy and a NH IC unit in Norway.
Although the aim, the set up, and the inclusion criteria of the two models had several similarities, a higher threshold for transferral of patients with a bad functional status to the Norwegian NH IC (with doctors present only at daytime) was probably applied, as compared to the Italian patients referred for hospital SAC. The Italian patients in general were more clinically instable, had higher medical needs, and the majority of them would probably be unable to be treated in a different settings other than a hospital. However, some of them, either with the best or the worst functional status might also have benefitted from treatment in different care facilities outside the hospital, as for example a NH IC ward (best status) or a NH palliative ward (worst status). Altogether this suggests that different postacute alternatives are needed for patients with different functional status and different medical needs, but more firm inclusion criteria’s might be considered to optimize the selection of patients.
ADL function, assessed by BI was the most important clinical factor predicting the ability to return to own home, both for the Italian and the Norwegian patients, and this is partly in accordance with other studies (32, 33). In the Italian patients, higher scores on GDS, implying depression, were also significantly negatively associated with the ability to return home, while this was not so for the Norwegian patients. The present study could not explain this difference, but other studies have demonstrated that older hospitalized patients with depressive symptoms are at higher risk of unfavorable outcome and mortality (34, 35).
A limitation of the present study is that we have only reported short term results while only follow up over time concerning mortality and autonomy can tell to what extent the patients are suited for and have benefit from the two different treatment modalities. We are planning to do such a study when the patients have been followed for 12 months. Secondly, two different PAC settings – in hospital and in NH were compared, thus the generalizability of the study may be limited. The strength of the study is that, as far as we know, no other study has earlier compared different PAC models in different countries.
We conclude that some caution should be taken when clinical outcomes from different countries and societies are compared, because end-points, like the ability to return to home and the use of NH, is influenced by health care and sociodemographic differences. Both the Italian hospital SAC model and the Norwegian NH IC model presented in this article are feasible and good alternatives, but more firm inclusion criteria based on knowledge about the long term clinical outcome of both patient groups may further optimize the selection of patients suitable for these different PAC options.
Funding: This work was funded by Western Norway Regional Health Authority. No commercial role was played in the design, execution, analyses, interpretation of data or writing of the study.
Conflict of interest: JFA, RR, SB, AC, AHR and MT have no conflicts of interests to declare.
References
1. Boyd CM, Landefeld CS, Counsell SR, Palmer RM, Fortinsky RH, Kresevic D, et al. Recovery of activities of daily living in older adults after hospitalization for acute medical illness. J Am Geriatr Soc 2008;56(12):2171-9.
2. Wong RY, Miller WC. Adverse outcomes following hospitalization in acutely ill older patients. BMC geriatr 2008;8:10.
3. Gill TM, Gahbauer EA, Han L, Allore HG. Factors associated with recovery of prehospital function among older persons admitted to a nursing home with disability after an acute hospitalization. J Gerontol A Biol Sci Med Sci 2009;64(12):1296-303.
4. Young J, Green J, Forster A, Small N, Lowson K, Bogle S, et al. Postacute care for older people in community hospitals: a multicenter randomized, controlled trial. J Am Geriatr Soc 2007;55(12):1995-2002.
5. Lee WJ, Peng LN, Cheng YY, Liu CY, Chen LK, Yu HC. Effectiveness of short-term interdisciplinary intervention on postacute patients in Taiwan. J Am Med Dir Assoc 2011;12(1):29-32.
6. Martin GP, Hewitt GJ, Faulkner TA, Parker H. The organisation, form and function of intermediate care services and systems in England: results from a national survey. Health Soc Care Community 2007;15(2):146-54.
7. Garasen H, Windspoll R, Johnsen R. Intermediate care at a community hospital as an alternative to prolonged general hospital care for elderly patients: a randomised controlled trial. BMC Public Health. 2007;7:68.
8. Ariss SM, Enderby PM, Smith T, Nancarrow SA, Bradburn MJ, Harrop D, et al. Secondary analysis and literature review of community rehabilitation and intermediate care: an information resource. Health Serv and Deliv Res 2015; No 3. 1. Southampton (UK)
9. Bachmann S, Finger C, Huss A, Egger M, Stuck AE, Clough-Gorr KM. Inpatient rehabilitation specifically designed for geriatric patients: systematic review and meta-analysis of randomised controlled trials. BMJ 2010;340:c1718.
10. Gindin J, Walter-Ginzburg A, Geitzen M, Epstein S, Levi S, Landi F, et al. Predictors of rehabilitation outcomes: a comparison of Israeli and Italian geriatric post-acute care (PAC) facilities using the minimum data set (MDS). J Am Med Dir Assoc 2007;8(4):233-42.
11. Garasen H, Windspoll R, Johnsen R. Long-term patients’ outcomes after intermediate care at a community hospital for elderly patients: 12-month follow-up of a randomized controlled trial. Scand J Public Health 2008;36(2):197-204.
12. Chen LK, Chen YM, Hwang SJ, Peng LN, Lin MH, Lee WJ, et al. Effectiveness of community hospital-based post-acute care on functional recovery and 12-month mortality in older patients: a prospective cohort study. Ann Med 2010;42(8):630-6.
13. Kilgore C. Why intermediate care services need to be refreshed. Nurs Older People 2014;26(3):16-20.
14. Plochg T, Delnoij DM, van der Kruk TF, Janmaat TA, Klazinga NS. Intermediate care: for better or worse? Process evaluation of an intermediate care model between a university hospital and a residential home. BMC Health Services Res 2005;5:38.
15. Boffelli S, Cassinadri A, Mercurio F, Rozzini R, Trabucchi M. Le cure sub acuta fra ospedal e territorio: una nuova opportunita di cura geriatrica. J Gerontol. 2014;62:21-8.
16. Herfjord JK, Heggestad T, Ersland H, Ranhoff AH. Intermediate care in nursing home after hospital admission: a randomized controlled trial with one year follow-up. BMC Res Notes 2014;7(1):889.
17. Abrahamsen J, Haugland C, Nilsen R, Ranhoff AH. Predictors for return to own home and being alive at 6 months after nursing home intermediate care following acute hospitalization. Eur Geriatr Medi 2014;5:108-12.
18. Abrahamsen J, Haugland C, Nilsen R, Ranhoff AH. Three different outcomes in older community-dwelling patients receiving intermediate care in nursing home after acute hospitalization. J Nutr Health Aging. 2015;In press.
19. Mahoney FI. Functional Evaluation: the Barthel Index. Md State Med J. 1965;14:108-12.
20. Folstein MF, Folstein SE, McHugh PR. «Mini-mental state». A practical method for grading the cognitive state of patients for clinicians. J Psychiatr Res 1975;12:189-98.
21. Yesavage JA. Geriatric Depression Scale. Psychopharmacol Bull 1988;24(4):709-11.
22. Tinetti ME. Performance-oriented assessment of mobility problems in elderly patients. J Am Geriatr Soc 1986;34(2):119-26.
23. Parmelee PA, Thuras PD, Katz IR, Lawton MP. Validation of the Cumulative Illness Rating Scale in a geriatric residential population. J Am Geriatr Soc 1995;43(2):130-7.
24. Blaylock A, Cason CL. Discharge planning predicting patients’ needs. J Gerontolo Nurs 1992;18(7):5-10.
25. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. The Gerontologist. 1969;9(3):179-86.
26. Lanctot KL, Hsiung GY, Feldman HH, Masoud ST, Sham L, Herrmann N. Assessing the validity of deriving clinical dementia rating (CDR) global scores from independently-obtained functional rating scale (FRS) scores in vascular dementia with and without Alzheimer’s disease. Int J Geriatr Psychiatry. 2009;24(10):1174-6.
27. Strobel CEK, Engedal K. MMSE-NR. Norwegian Revised Mini Mental Status Evaluation. Revised and Expanded Manual. Norwegian Centre for Ageing and Health, Oslo, Norway, 2008.
28. Guigoz Y, Lauque S, Vellas BJ. Identifying the elderly at risk for malnutrition. The Mini Nutritional Assessment. Clin Geriatr Med 2002;18(4):737-57.
29. Wilson CB. An updated Declaration of Helsinki will provide more protection. Nature Med 2013;19(6):664.
30. Ward D, Drahota A, Gal D, Severs M, Dean TP. Care home versus hospital and own home environments for rehabilitation of older people. The Cochrane database of systematic reviews. 2008(4):CD003164.
31. Young J. The development of intermediate care services in England. Arch Gerontol Geriatr 2009;49 Suppl 2:S21-5.
32. Bellelli G, Magnifico F, Trabucchi M. Outcomes at 12 months in a population of elderly patients discharged from a rehabilitation unit. J Am Med Dir Assoc 2008;9(1):55-64.
33. Sleiman I, Rozzini R, Barbisoni P, Morandi A, Ricci A, Giordano A, et al. Functional trajectories during hospitalization: a prognostic sign for elderly patients. J Gerontol A Biol Sci Med Sci 2009;64(6):659-63.
34. Covinsky KE, Kahana E, Chin MH, Palmer RM, Fortinsky RH, Landefeld CS. Depressive symptoms and 3-year mortality in older hospitalized medical patients. Ann Intern Med 1999;130(7):563-9.
35. Cullum S, Metcalfe C, Todd C, Brayne C. Does depression predict adverse outcomes for older medical inpatients? A prospective cohort study of individuals screened for a trial. Age Ageing. 2008;37(6):690-5.