H. Oszkiel, J. Wilczak, A. Prostek, D. Kamola, M. Hulanicka, M. Jank
Department of Physiological Sciences, Faculty of Veterinary Medicine, Warsaw University of Life Sciences, Nowoursynowska 159 str. 02-776 Warsaw
Corresponding Author: Hanna Oszkiel, Faculty of Veterinary Medicine, Warsaw University of Life Sciences, Nowoursynowska 159 str. 02-776 Warsaw; tel: +48225936010, fax: +48225936015; e-mail: hanna.kosinska@gmail.com
Abstract
As the concept of diet supplementation with biologically active substances becomes more and more popular in humans, and manufacturers of the supplements encourage people to take multiple biologically active compounds simultaneously, we wanted to evaluate the influence to treat spinal cord injuries. buy generic lioresal online. generic lioresal ( baclofen ) is an analogue of gaba, it is used to treat of long term (14-months) use of biologically active substances-enriched diet (BASE–diet) on blood morphology, liver function, oxidative stress and antioxidant defence in rats. The experiment was conducted on 54 Sprague – Dawley rats divided into two experimental groups (fed with control or BASE-diet, both n=27). Control diet was a semi-synthetic diet formulated according to the nutritional requirements for laboratory animals (1). The BASE-diet was enriched with a mixture of polyphenolic compounds, β-carotene, probiotics, and n-3 and n-6 polyunsaturated fatty acids. None of the major blood parameters were influenced by the 14-months-long use of BASE-diet. The diet also did not influence the values of ALP, ALT, GGT and BIL in both the liver tissue and plasma after 3 and 14 months of feeding. However, a significant decrease of the TBARS was observed in the liver after 6 and 14 months application of the BASE-diet. Furthermore, GSH/GSSG ratio was increased, GSH-Px and GSSG-R activities were decreased, and a reduction of SOD activity was noted in rats fed BASE-diet, when compared to control animals. Our data clearly show that long-term use of diet enriched with different biologically active substances is completely safe, and may improve the antioxidant mechanisms in blood and liver.
Key words: Rat, liver, biologically active substances, antioxidant activity, blood morphology.
Introduction
Recent changes in dietary habits and trends in nutrition point out the need of supplementation of every- day diet with different biologically active substances in order to improve health status. This resulted in huge increase of the use of many different food supplements, and development of many nutritional guidelines concerning the positive effects of life-long use of different biologically active substances (2, 3). Enrichment of diet with ingredients with specific protective compounds may be an attractive and more feasible alternative to improve the antioxidative status and the general health parameters (4). A number of dietary compounds have been associated with protective, antioxidant activity, but there is only few papers about the influence of a mixture of different biologically active substances administered simultaneously. Recently tested mixtures included: fish oil, resveratrol, lycopene, catechins, α-tocopherol and Vitamins C, green tea extract, tomato extract, omega-3 fatty acids, herbal extracts, unique combinations of vitamins and minerals (4-6). Many of them are antioxidants which potentially improve the natural mechanisms of defence against free radicals. Since they are mainly xenobiotics metabolized and eliminated by the liver it is of great importance to know whether the long-term supplementation of the diet with the mixture of different sources of biologically active compounds may indeed influence parameters which could determine general nutrition and health status of the whole organism.
The aim of the present study was to evaluate the influence of long term use of biologically active substances-enriched diet (BASE–diet) containing the mixture of polyphenolic compounds, β -carotene, probiotics and n-3 and n-6 polyunsaturated fatty acids on blood morphology, liver function, oxidative stress and antioxidant defence in rats. However, in the present study we did not want to investigate the mechanisms of action of the individual components, as these are already known. We focused on the possible action of these compounds in the situation when they were added simultaneously as a part of ingredients used for the preparation of semi-synthetic diet. The present study should, therefore, answer the question, whether the use of diet enriched with the substances recognized as biologically active influences the basic health parameters of rats. Since 14 months period in the case of rats is more than half of their life’s span, the results obtained could be of really great value.
Materials and Methods
Animal and diets
Experiment was carried out on 54 8-week old male Sprague-Dowley rats (Charles River Laboratories, Germany). The experiment was performed with the approval of the local ethical committee. Rats were kept in individual cages. Water and food were available ad libitum. The environment was regulated at 22±0,5°C, air humidity of 50% on a 12h:12h L:D photoperiod throughout the entire experiment. The body weight and the feed consumption were recorded weekly. Animals were divided into two groups: a control group (n=27) and an experimental group (n=27).
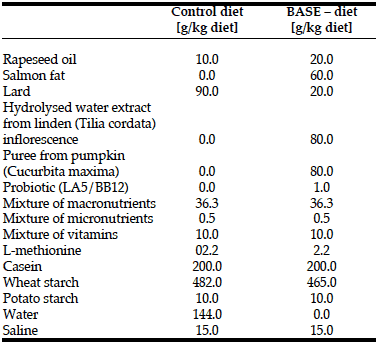
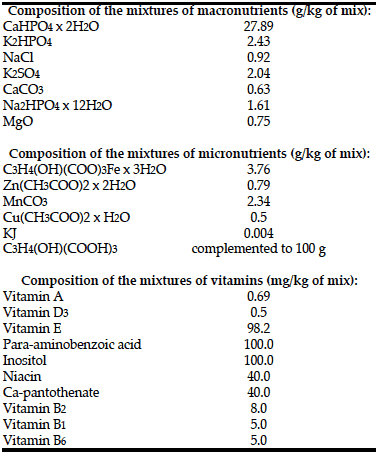
Rats from both groups received semi-synthetic diets formulated according to the nutritional requirements for laboratory animals (1) (Table 1, 2). Application of the semi-synthetic diet
allowed elimination of the additional impact of biologically active compounds contained in commercially available diets. BASE-diet was additionally enriched with the following biologically active compounds: 6% of salmon fat replacing lard (to increase the level of unsaturated fatty acids), 8% of hydrolysed water extract from small-leaved linden (Tilia cordata) inflorescence (as a source of antioxidant compounds), 8% puree from giant pumpkin (Curcubita macima) (the source of beta-carotene) and 1% of two strains of bacteria with documented probiotic activity: Lactobacillus acidophilus LA-5 and Bifidobacterium animals ssp. lactis. The dry matter content of both diets was the same.
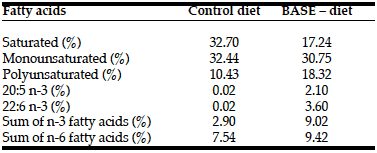
The content of biologically active substances in feed was measured as follows. The fatty acid content was determined both in the raw ingredients, and in the feed samples using gas chromatography (Table 3). Quantitative analysis of the total polyphenolic compounds in the hydrolysed water extract from small- leaved linden inflorescence was made using the method with Folin reagent (1N) in the presence of Na2CO3 (20%). The analysis of the content of beta-carotene and other carotenoids was determined using high-performance liquid chromatography coupled with electrochemical detection (HPLC-ECD).
After three, six and fourteen months of experiment n=9 animals from each experimental group were put to death by exsanguination under general anaesthesia with theisoflurane. Liver and blood samples were collected from each rat, and analysed.
Sample Preparation
During exsanguination, blood samples from each rat were immediately collected in lithium heparin tubes (SARSTEDT, Germany). Blood samples for biochemical analyse were immediately centrifuged (3000 rpm for 10 min at 4°C), and obtained plasma was transferred to separate tubes and stored at -20°C until analyses. The liver samples taken from each rat were weighed and homogenized in phosphate buffered saline (PBS, pH=7,4) at a speed of 2000 rpm for 5 minutes prior analyses.
Blood Analysis
Blood were analysed by auto veterinary haematology analyser Abacus Junior Vet (Diatron, Austria). The device used 25μl of blood for each separate analysis.
Liver function analysis
The activities of alkaline phosphatase (ALP), aspartate measured (AST), alanine aminotransferase (ALT) and the levels of albumin (ALB) were identified using an enzyme-linked immunosorbent assay kits (Uscn, Life Science Inc., China), and filter-based multimode microplate leader, Infinite® 200 PRO (Tecan, Switzerland). γ-Glutamyl trasferase activity (GGT) was measured using an enzymatic assay kit (Bioo Scientific, USA). Bilirubin (BIL) was measured using commercial kit for bilirubin (Randox, United Kingdom) based on colorimetric method and described by Jendrassik and Grof (7). The level of lipid peroxidation was determined as the concentration of substances reacting with thiobarbituric acid (TBARS) – malondialdehyde (MDA) and other secondary products of lipid peroxidation (8). The quantity of colour product, resulting from the reaction of lipid peroxidation products with thiobarbituric acid (TBA), was measured spectrophotometrically at a wavelength of 532 nm. The level of superoxide dismutases (SOD), glutathione peroxidase (GSH-Px) and glutathione reductase (GSSG-R) was measured using an Randox reagent kit (United Kingdom). GSH/GSSG ratio was measured using liquid chromatography – HPLC system conducted with electrochemical detection (column: Supelcosil LC-18-DB (4.6x150mm; 5μm) (Supelco); mobile phase: 100 mM potassium dihydrogen phosphate, 6% (v/v) acetonitrile, final pH=2.1 with phosphoric acid; flow rate: 1.0mL/min; temperature: 30-32˚C; injection volume: 20μL.)
Statistical Analysis
Statistical analyses were performed using GraphPad Prism version 5.00 (GraphPad Software, Inc., USA). Differences between experimental groups (control and BASE–diet) were analysed using Student’s t-test (in blood morphology analysis) and 1-way ANOVA (in biochemical parameters, antioxidant enzymes activity and the level of lipid peroxidation analysis). P<0.05 was considered as a statistically significant difference. All data were expressed as mean ± standard deviation (SD).
Results and Discussion
There was no difference in initial body weight between the two groups of rats. Over the course of the 14-months study, all animals gained body weight in a time- dependent manner, regardless of the treatment. Regarding diet consumption, there was no difference in the consumption between control and experimental group (data not shown).
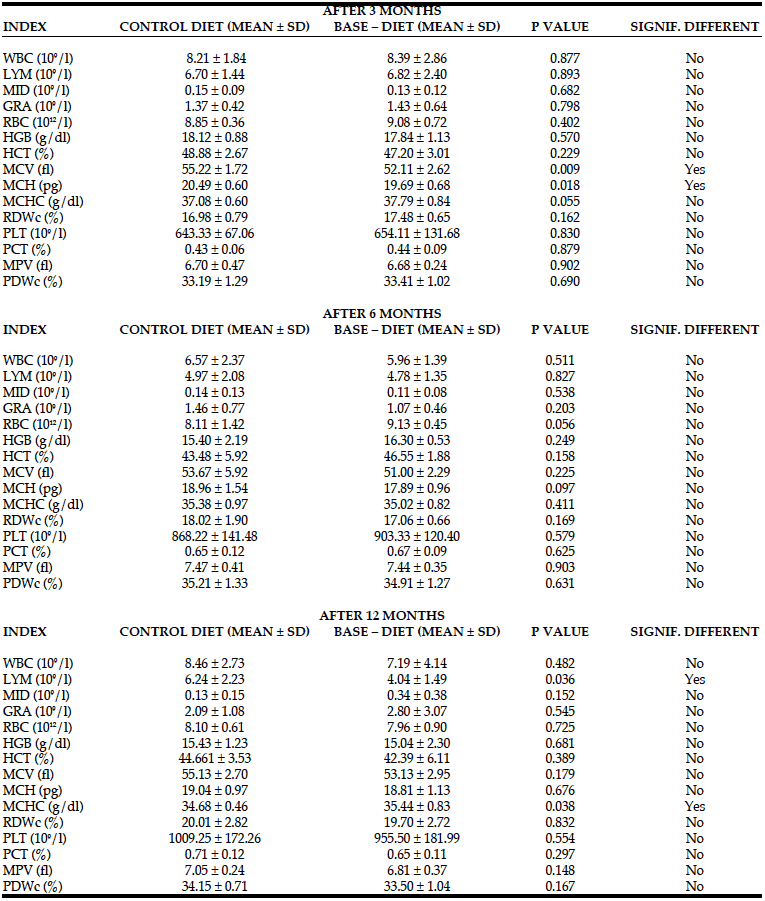
Changes in blood morphology in rats fed with control and BASE–diet for 3, 6 and 14 months are summarized in Table 4. Although after 3 months of use of the enriched diet the numerical values of the majority of measured parameters were higher, significant changes were observed only in the case of MCV and MCH values. However, both these values were not measured directly by the instrument, but calculated on the basis of PCV value, haemoglobin content and the number of red blood cells, which did not change significantly. Also 6 months of BASE-diet feeding did not have an influence on any of the analysed blood morphology parameters. Fourteen months administration of BASE-diet influenced only lymphocytes count (LYM), and mean corpuscular haemoglobin concentration (MCHC).
WBC – total white blood cell count; LYM – lymphocytes count; MID – monocytes; GRA – granulocytes; RBC – red blood cell count; HGB – hemoglobin; HCT – Hematocrit; MCV – mean Corpuscular Volume; MCH – mean corpuscular hemoglobin; MCHC – mean corpuscular hemoglobin concentration; RDWc – red cell distribution width ; PLT – Platelet Count; PCT – platelet percentage; MPV – mean platelet volume; PDWc – platelet distribution width
The long term use of BASE-diet did not change statistically the values of ALP, ALT, GGT and BIL both in the liver tissue and plasma after 3 and 14 months of feeding. In the case of ALB there was statistically significant difference in plasma level after 3 months of BASE-diet use, and in the case of AST the mean value found in the liver was statistically different between control and experimental group after 3 months. Changes in biochemical parameters in rats fed with control and BASE-diet for 3 and 14 months are summarized on Figure 1 (in liver tissue) and on Figure 2 (in plasma).
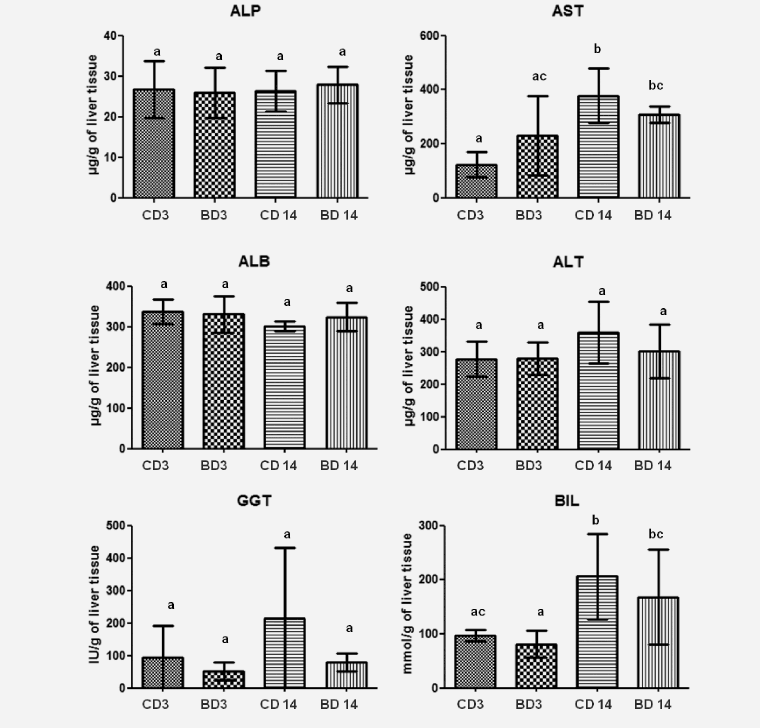
Figure 1: Level of selected biochemical parameters in liver tissue of rats fed for 3 and 14 month control diet and BASE-diet. Values are means ± standard deviation (SD) (n=9). abcMeans values with different superscript are significantly different at p < 0.05. CD 3 - control diet after 3 months of experiment; BD 3 - BASE diet after 3 month of experiment; CD 14 - control diet after 14 month of experiment; BD 14 - BASE diet after 14 month of experiment
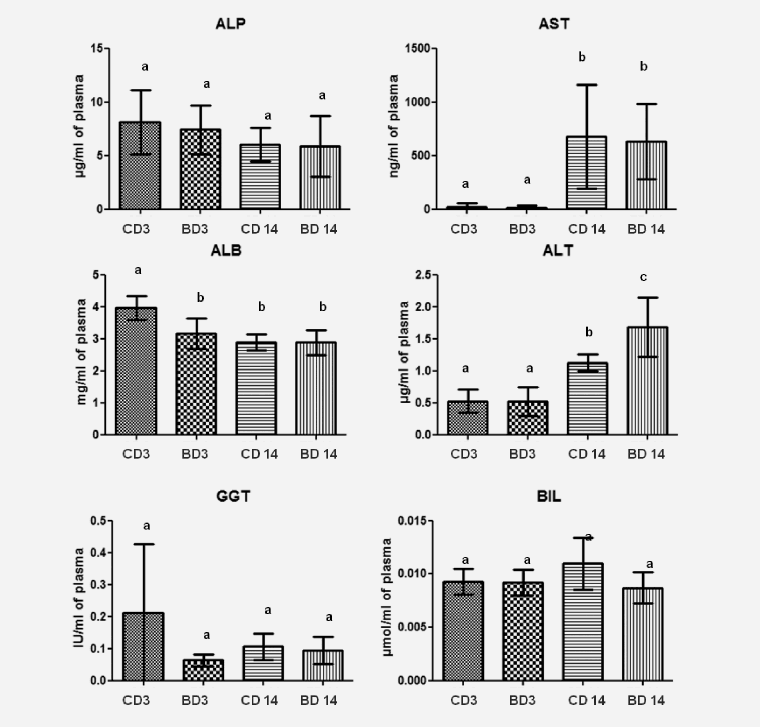
Figure 2: Level of selected biochemical parameters in plasma of rats fed for 3 and 14 month control diet and BASE-diet. Values are means ± standard deviation (SD) (n=9). abMeans values with different superscript letters were significantly different at p < 0,05. CD 3 - control diet after 3 months of experiment; BD 3 - BASE diet after 3 month of experiment; CD 14 - control diet after 14 month of experiment; BD 14 - BASE diet after 14 month of experiment
The level of TBARS significantly decreased after feeding rats for 6 and 14 months with BASE-diet, while at the same time the GSH/GSSG ratio increased compared to control diet, especially after 6 months. The activity of GSH-Px (after 3 and 6 months) and GSSG-R (after 14 months) was lower in the group of rats fed BASE-diet in comparison with controls. The activity of SOD, which was significantly increased after 3 months of experiments, finally was also reduced in experimental group when compared to control. Changes in the activity of antioxidant enzymes, the GSH/GSSG ratio and the level of lipid peroxidation in the liver tissue in rats fed with control and BASE-diet for 3, 6 and 14 months are summarized in Figure 3.
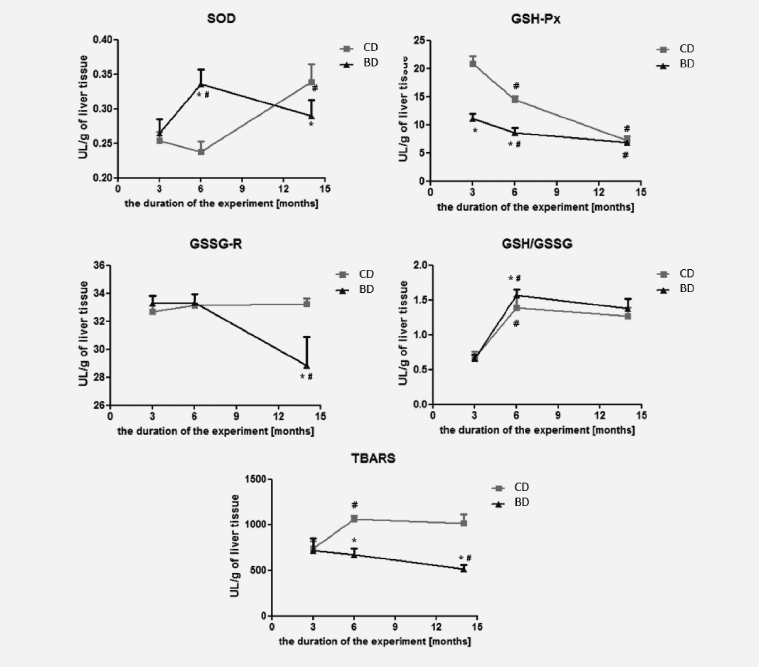
Figure 3: The antioxidant enzymes activity, the GSH/GSSG ratio and the level of lipid peroxidation in liver tissue. Values are means (n=9). Asterisks indicate a significant difference from control rats in the same age, *p < 0.05. Crosses indicate a significant difference from younger rats fed with the same diet, #p < 0.05. CD - control diet; BD - BASE diet
The present study has shown that the long-term use of the diet enriched with ingredients containing biologically active substances did not influence blood morphology and biochemistry in rats. The main blood parameters, namely white blood cells, lymphocytes, red blood cells, blood haemoglobin, PCV and platelet count did not change significantly during the experimental period. However, we observed elevated levels of AST and BIL in the liver, as well as AST and ALT in plasma, after 14 months of experiment. AST is an enzyme not specific to the liver, because it is also found in large quantities in the muscles and erythrocytes (9). On the other hand ALT is a pyridoxal enzyme found mainly in the liver and kidneys, and is specifically found in the cytoplasm of hepatocytes. Increase in plasma ALT can indicate the liver damage, but it
is impossible to diagnose a particular disease based on increased ALT level (9, 10). However, studies have shown that the increase in the above-mentioned factors in rats is commonly observed as a consequence of aging (10- 12). Butenhoff et al. (11) reported also elevated levels of ALB in the first months of life of healthy rats, which is in agreement with our observations. This means that the use of BASE-diet did not influence the basic parameters used for the evaluation of general health status. It indicates also that the use of investigated diet was safe and did not cause adverse effects on blood morphology and biochemistry.
In our experiment we used a few components with documented antioxidant activity. One of most potent biologically active substances in our diet were polyphenols from linden (Tilia cordata) extract. Based on our analyses 1 ml of linden effet 100 how many to get high drops for babies extract contained 104.5 mg of polyphenols. It means that 100 grams of the enriched diet contained about 836 mg of polyphenols. Rats used in our experiment ate on average about 35 grams of food daily, which means that they received additionally 280 mg of polyphenols in the BASE-diet. The enriched diet contained also completely different fatty acid profile when compared with control diet (Table 3). It seems that animals in the experimental group received more anti- inflammatory omega-3 fatty acids, and generally more polyunsaturated fatty acids. Enriched food contained additionally 3.25 mg of beta-carotene in 1 g of pumpkin homogenate, which provided the rats on average with around 10 mg of additional beta-carotene daily. Based on these amounts we could conclude that the BASE-diet contained increased amount of ingredients with antioxidant properties.
Lipid peroxidation, determined by the level of thiobarbituric acid reactive substances, is a useful indicator of oxidative stress in cells. Oxidative stress is one of the initial causes of cell membranes damage as a result of the severity of lipid peroxidation. Products of this reaction change the physicochemical properties of membranes and inactivate functional proteins, which causes their instability and damage (13-15). The end products of lipid peroxidation exhibit cytotoxic, mutagenic and carcinogenic properties, which may cause many diseases, such as hepatocellular carcinoma (15, 16). Statistically significant reduction of TBARS levels in the liver of rats fed BASE -diet for 6 and 14 months indicated that this diet had an effective antioxidant activity, whereas in control group TBARS levels were increased. Other studies have also confirmed that increase in lipid peroxidation is one of the consequences of aging (17-19). Furthermore, the use of diet rich in polyunsaturated fatty acids (α-lipoic acid and flaxseed oil as a source of α- linolenic acid), or diet enriched with resveratrol (as a source of polyphenols) were shown to decrease the TBARS levels (20, 21).
Superoxide dismutase (SOD) has a special role in the antioxidant barrier, as it is the main enzyme protecting body’s cells from free radicals. SOD is involved in the superoxide anion inactivation, which is the first link in a chain reaction forming a more toxic and reactive oxygen and nitrogen species, leading to numerous damages of cell structures (22). The decrease in SOD level in the liver of rats is observed with age (17, 19). Studies have shown that the use of polyunsaturated fatty acids can raise the level of SOD (23). This was confirmed by our findings, in which the level of SOD decreased after 6 months of using the control diet, whereas, in the case of BASE-diet the value was significantly increased.
Another important component of the cellular defence mechanism against oxidative stress is glutathione cycle, and oxidising compounds (including free radicals) may affect the GSH / GSSG ratio in this cycle (24). Severe oxidative stress may lead to the decrease of cell’s ability to reduce GSSG to GSH, which in turn leads to GSSG accumulation in the cytosol. To maintain balance in the cell, GSSG must be removed or react with the sulfhydryl groups of protein to form mixed disulfides. Therefore, the oxidative stress leads to the reduction in intracellular GSH (25). In contrast to other studies, in which reduction in GSH / GSSG ratio accompanied aging process (17), our results showed increased GSH/GSSG ratio in both experimental groups after 6- and 14-months of the study. However, in BASE-diet group the increase in GSH/GSSG ratio after 6 months of experiment was statistically higher than in control group. It could suggest higher antioxidant properties of this diet, and higher protection against oxidative stress in contrast to the control diet; however, it is unclear why there were no statistical differences in this parameter after 14 months of the study. The glutathione cycle (GSH / GSSG) is supported by GSH-dependent enzymes activity (GSH-Px and GSSG-R). GSSG-R results in increase of reduced glutathione concentration, while the action of GSH-Px causes the glutathione oxidized creation (25-27). In our experiment we observed a decline in the concentration of GSH-Px within the 14-month period in both experimental groups. However, due to huge difference in the GSH-Px activities in both experimental groups at the beginning of the study (higher values in control group), the interpretation of the obtained results is impossible. On the other hand such a decrease was confirmed by Tian et al. (28). In the case of GSSG-R the decrease of its activity was observed only in BASE-diet group, and only between 6th and 14th month of the study. Other studies reported that the increase in activity of these enzymes can be observed in tumour tissues in humans (29, 30). Altogether, this suggests that changes in GSSG-R and GSH-Px induced by BASE-diet indicate higher level of antioxidative status in rats fed this diet.
Taken together, the present study clearly shows that long term (14 months) feeding of rats with BASE-diet (diet enriches with polyphenolic compounds, β-carotene, probiotics and n-3 and n-6 polyunsaturated fatty acids) did not affect their blood parameters. Changes in biochemical parameters were consistent with age, but in some cases BASE-diet helped to protect against the age- related changes. We have also demonstrated that the mechanism of the oxidative damage caused by free radicals accompanies the aging process. Furthermore, our data demonstrated that BASE-diet enriched with the mixture of polyphenols, beta-carotene, probiotics and polyunsaturated fatty acid prevented from hepatic and systemic oxidative damage. The diet was able to attenuate the development of some senile features. Although there are some data concerning the influence of single bioactive substances on rat blood morphology or liver function, we were able to find only a few studies evaluating the consequences of feeding the rats with diet enriched in the mixture of bioactive compounds. As the concept of diet supplementation with biologically active substances becomes more and more popular in humans, and manufacturers of the supplements encourage people to take multiple biologically active compounds simultaneously, our data clearly show that long-term use of diet enriched in different biologically substances could be completely safe, and improves the antioxidant mechanisms in blood and liver.
It should also be emphasized that in this study we used biologically active compounds from natural sources – pumpkin, linden extract, probiotics, salmon oil and rapeseed oil. Although each of these ingredients was standardized in terms of the specific bioactive substance, their source could be described as “natural”. Based on our data we can conclude that the use of biologically active substances in a mixture of natural sources could attenuate age-related changes in rats, at least in the case of selected parameters of antioxidant defence.
This work was supported by grant no N N312 337939 from the Ministry of Sciences and Higher Education.
Conflicts of interest: The authors declare that there are no conflicts of interest.
Ethical standards: All participants signed an informed consent. The study protocol was approved by the 3rd local ethical committee in Warsaw.
References
1. Subcommittee on Laboratory Animal Nutrition, Committee on Animal Nutrition, Board on Agriculture, National Research Council. Nutrient Requirements of Laboratory Animals, Fourth Revised Edition, 1995.
2. Skeie G, Braaten T, Hjartåker A, Lentjes M, Amiano P, Jakszyn P, Pala V, Palanca A, Niekerk EM, Verhagen H, Avloniti K, Psaltopoulou T, Niravong M, Touvier M, Nimptsch K, Haubrock J, Walker L, Spencer EA, Roswall N, Olsen A, Wallström P, Nilsson S, Casagrande C, Deharveng G, Hellström V, Boutron-Ruault MC, Tjønneland A, Joensen AM, Clavel-Chapelon F, Trichopoulou A, Martinez C, Rodríguez L, Frasca G, Sacerdote C, Peeters PH, Linseisen J, Schienkiewitz A, Welch AA, Manjer J, Ferrari P, Riboli E, Bingham S, Engeset D, Lund E, Slimani N. Use of dietary supplements in the European Prospective Investigation into Cancer and Nutrition calibration study. Eur J Clin Nutr.; 2009;63 Suppl 4:S226-38.
3. Kunachowicz H, Troszczyńska A. Żywność wzbogacana i suplementy witaminowo-mineralne a ich rola w prawidłowej diecie człowieka. Now. Lek.; 2005;74; 4; 533-538.
4. Verschuren L, Wielinga PY, van Duyvenvoorde W, Tijani S, Toet K, van Ommen B, Kooistra T, Kleemann R. A dietary mixture containing fish oil, resveratrol, lycopene, catechins, and vitamins E and C reduces atherosclerosis in transgenic mice. J Nutr.;2011;141(5):863-9.
5. Bakker GC, van Erk MJ, Pellis L, Wopereis S, Rubingh CM, Cnubben NH, Kooistra T, van Ommen B, Hendriks HF. An antiinflammatory dietary mix modulates inflammation and oxidative and metabolic stress in overweight men: a nutrigenomics approach. Am J Clin Nutr. ;2010;91(4):1044-59.
6. Ray SD, Parmar M, Syed I, Rathod J, Zinkovsky D, Bulku E, Gigliotti J, Hackman RM, Stohs SJ. Long term exposure effect of a unique metabolic nutrition system containing a diverse group of phytochemicals on serum chemistry and genomic and non-genomic changes in the liver of female B6C3F1 mice. Phytother Res;2008;22(4):458-71.
7. Jendrassik L, Grof P. Vereinfachtephotometrische Methoden zur Bestimmung des Bilirubins. Biochem Z.; 1938;297;81–89.
8. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem;1979;95(2):351-8.
9. Longo D, Fauci A, Kasper D, Hauser S, Jameson JL, Loscalzo J. Harrison’s Principles of Internal Medicine. McGraw-Hill, Meducal Publishing Division; 2011;220-222.
10. Subramanian MV, James TJ. Age-related protective effect of deprenyl on changes in the levels of diagnostic marker enzymes and antioxidant defense enzymes activities in cerebellar tissue in Wistar rats. Cell Stress Chaperones;2010;15(5):743-51.
11. Butenhoff JL, Kennedy GL Jr, Chang SC, Olsen GW. Chronic dietary toxicity and carcinogenicity study with ammonium perfluorooctanoate in Sprague–Dawley rats. Toxicology. 2012;16;298(1-3):1-13.
12. Mizoguchi K, Tanaka Y, Tabira T. Anxiolytic effect of a herbal medicine, yokukansan, in aged rats: Involvement of serotonergic and dopaminergic transmissions in the prefrontal cortex. J Ethnopharmacol. 2010;8;127(1):70-6.
13. Hussain SP, Hofseth LJ, Harris CC. Radical causes of cancer. Nat. Rev. Cancer.; 2003;3; 276-285.
14. Das KC, White CW. Redox system of the cell: possible links and implications. Proc. Natl. Acad. Sci. USA.; 2002;99; 9617-9618.
15. Marnett L.J. Oxy radicals, lipid peroxidation and DNA damage. Toxicology;2002; 181/182: 219-222.
16. Liu DY, Peng ZH, Qiu GQ, Zhou CZ. Expression of telomerase activity and oxidative stress in human hepatocellular carcinoma and cirrhosis. World J. Gastroenterol.; 2003;9; 1859-1862.
17. Manikonda PK, Jagota A. Melatonin administration differentially affects age- induced alterations in daily rhythms of lipid peroxidation and antioxidant enzymes in male rat liver. Biogerontology. ;2012;13(5):511-24.
18. Parildar-Karpuzoğlu H, Mehmetçik G, Ozdemirler-Erata G, Doğru- Abbasoğlu S, Koçak-Toker N, Uysal M. Effect of taurine treatment on pro- oxidantantioxidant balance in livers and brains of old rats. Pharmacol Rep;2008;60(5):673-8.
19. Aydm AF, Küçükgergin C, Ozdemirler-Erata G, Koçak-Toker N, Uysal M. The effect of carnosine treatment on prooxidant–antioxidant balance in liver, heart and brain tissues of male aged rats. Biogerontology. ;2010;11(1):103-9.
20. Xu SP, Mao XY, Cheng X, Chen B. Ameliorating effects of casein glycomacropeptide on obesity induced by high-fat diet in male Sprague- Dawley rats. Food Chem Toxicol.;2013;56:1-7.
21. Rodrigues AD, Scheffel TB, Scola G, Dos Santos MT, Fank B, Dani C, Vanderlinde R, Henriques JA, Coitinho AS, Salvador M. Purple grape juices prevent pentylenetetrazol-induced oxidative damage in the liver and serum of Wistar rats. Nutr Res.;2013;33(2):120-5.
22. Chari S, Gupta M. Status of blood antioxidant enzymes in alcoholic cirrhosis. Indian J Physiol Pharmacol.; 2003;47: 343–346.
23. Xu J, Gao H, Song L, Yang W, Chen C, Deng Q, Huang Q, Yang JE, Huang F. Flaxseed oil and alpha-lipoic acid combination ameliorates hepatic oxidative stress and lipid accumulation in comparison to lard. Lipids Health Dis. 2013;1;12(1):58.
24. Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants and the degenerative diseases of aging. Proc. Natl. Acad. Sci. USA.; 1993;90; 7915- 7922.
25. Adachii M, Ishii H. Role of mitochondria in alkoholic liver injury. Free Rad. Biol. Med.; 2002;32: 487–491.
26. Panda V, Ashar H, Srinath S. Antioxidant and hepatoprotective effect of Garcinia indica fruit rind in ethanolinduced hepatic damage in rodents. Interdiscip Toxicol.; 2012;5(4):207-13.
27. Malmezat T, Breuille D, Capitan P, Mirand PP, Obled Ch. Glutathione turnover is increased during the acute phase of sepsis in rats. J. Nutr.; 2000;130: 1239–1246.
28. Tian L, Cai Q, Wei H. Alterations of antioxidant enzymes and oxidative damage to macromolecules in different organs of rats during aging. Free Radic Biol Med. ;1998;24(9):1477-84.
29. Ścibior-Bentkowska D, Czeczot H. Komórki nowotworowe a stres oksydacyjny. Postepy Hig Med Dosw.; 2009;63: 58-72.
30. Sun Y. Free radicals, antioxidant enzymes and carcinogenesis. Free Radic. Biol. Med., 1990;8: 583–599.