H. Talkad1, Y. Chen2, A.P. Bress3, J.B. Langbaum2, P.N. Tariot1,2, J.J. Pruzin1,2
1. University of Arizona College of Medicine – Phoenix, Phoenix, AZ, USA; 2. Banner Alzheimer’s Institute, Phoenix, AZ, USA; 3. University of Utah, Salt Lake City Utah
Corresponding Author: Harshita Talkad, University of Arizona College of Medicine – Phoenix, Phoenix, AZ, USA, htalkad@arizona.edu
J Aging Res & Lifestyle 2024;13:126-134
Published online December 16, 2024, http://dx.doi.org/10.14283/jarlife.2024.19
Abstract
BACKGROUND: Dementia disproportionately affects Hispanic communities, which may be partially attributable to disparities in resources to address modifiable risk factors. Addressing risk factors at younger ages would likely confer greater benefit than at older ages. Interest among Hispanic and younger persons participating in a dementia prevention program is unknown.
OBJECTIVES: To understand knowledge of dementia risk factors and attitudes toward prevention program participation among Arizona residents.
DESIGN: Cross-sectional study.
SETTING: Online survey conducted of Arizona residents in either English or Spanish between July 13, 2021 and August 2, 2021.
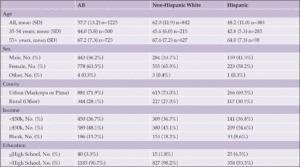
PARTICIPANTS: 1,303 persons age 35 and older; 332 (25.5%) were Hispanic.
MEASUREMENTS: Survey questions about knowledge of dementia risk factors and dementia prevention program interest. Comparisons between Hispanic and non-Hispanic White and younger and older respondents were made using chi-squared tests.
RESULTS: Overall, 30.7% of respondents were aware of any risk factors that increased risk for dementia with no differences between Hispanic and non-Hispanic White respondents. 76.4% of all respondents were “very” or “somewhat” interested in a dementia prevention program, interest was significantly higher in Hispanic (83.0% vs 73.3% “very” or “somewhat interested,” X2 (3, N=1226) = 14.8, p=0.002) and younger respondents (82.2% vs 72.1% “very” or “somewhat” interested X2 (1, N=1302) = 20.0, p<0.001).
CONCLUSION: General knowledge of risk factors for dementia is low, contrasting with high interest in a prevention program. Interest is higher in Hispanic and younger persons compared with older or non-Hispanic White persons. A dementia prevention program accessible to younger and Hispanic populations could help narrow dementia outcome disparities.
Key words: Alzheimer’s disease, health equity, risk factor.
Introduction
Alzheimer’s disease (AD), the most common cause of dementia, results from a long and complex pathophysiological process that begins at least two decades before the appearance of symptoms (1). While the accumulation of beta-amyloid (Aβ) plaques followed by hyperphosphorylated tau-containing neurofibrillary tangles define AD and are likely largely responsible for symptoms, the degree to which, and precisely how the presence of plaque and tangle pathology influence cognitive and functional symptoms remain incompletely understood. Many additional factors significantly influence the absolute risk and age of onset of both AD and AD-related dementias (AD/ADRD), many of which are modifiable (2). Previous studies highlight the importance of these risk factors, estimating that about 40% of dementia cases are attributable to them, and thus potentially preventable if fully addressed (2). Physical activity slows cognitive decline (3, 4) and lowers risk for AD/ADRD (5, 6). Sleep disturbances in middle age increase risk of dementia (7, 8). Loneliness increases risk, while strong social engagement is protective (9). Although recent evidence is mixed, eating a Mediterranean diet may lower risk (10, 11). Avoiding smoking (12), moderating alcohol consumption, treating hearing loss, obesity, and depression, and avoiding traumatic brain injury and polluted air also reduce the risk of developing dementia later in life (2, 10).
While risk factor modification in older adults is important in addressing AD/ADRD, epidemiological evidence shows that midlife may represent a crucial window to address many risk factors. For example, for diabetes and hypertension, the longer one has the condition and the more poorly controlled, the greater the later life dementia risk (13-15). Early intervention on modifiable risk factors will likely be more effective in reducing dementia risk compared to interventions at ages more proximate to a typical dementia diagnosis.
Next, interventions accessible to minoritized communities could help narrow AD/ADRD outcome disparities. Unaddressed modifiable risk factors and inequities in social determinants of health likely play an important role in driving disparities. Black and Hispanic persons are 1.5-2 times more likely than non-Hispanic persons to develop AD/ADRD (16). They have less brain Aβ (17) but more cerebral small vessel disease (18), indicative of differences in the etiology of AD/ADRD among ethnicities, likely reflecting the degree to which conditions like hypertension and diabetes are adequately treated (14, 15, 19). Additionally, risk factors like diet and physical activity level vary in relationship to race and socioeconomic status, which may be due to differences in the built environment, including access to parks and quality food, and neighborhood walkability (20, 21).
A small number of cognitive health clinics aimed at providing care and recommendations to delay or even prevent the onset of dementia have been established to address AD/ADRD through optimization of modifiable risk factors, with early evidence suggestive of a benefit (22, 23). However, the focus of these programs is often on older individuals and does not emphasize including marginalized populations. While dementia prevention programs may provide the most benefit to younger persons and those in disadvantaged communities, the degree of interest in cognitive health clinics aimed at dementia prevention in general and attitudes about participation in such programs among younger persons and those in marginalized Hispanic communities are unknown. To address this knowledge gap, we surveyed Arizona residents age 35 and over about knowledge of modifiable risk factors for dementia and attitudes toward prevention programs to address risk factors. We next examined differences in responses between Hispanic and non-Hispanic White respondents as well as between younger and older respondents (age 35-54 vs 55 and over) to determine the interest and feasibility of establishing a cognitive health clinic to reduce dementia risk serving middle-aged residents, with an emphasis on Hispanic community participation, in Arizona.
Methods
Survey Data Collection
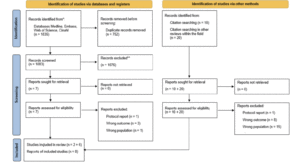
We partnered with SSRS, a professional market and survey research firm, to survey Arizona residents age 35 or older using an online self-administered questionnaire in either English or Spanish between July 13, 2021 and August 2, 2021. The questions were developed as a practical, exploratory approach to gather information about knowledge of AD/ADRD risk factors and attitudes toward participation in dementia prevention and research. The questions of interest are shown in Table 1. The study utilized third-party opt-in web unpaid panels with potential respondents being invited via email. Within the survey, respondents were screened to ensure that they met qualification criteria. Multiple panels were used to ensure adequate representation of hard-to-reach respondents, including Hispanic and Black households as well as those with income <$25,000 per year. Extensive program checks were conducted to ensure that skip patterns followed the design of the questionnaire, and the program was tested via desktop computer as well as mobile devices to ensure consistent visualization across devices prior to execution. The final program used various quality checks, including questions asking respondents to select a specific answer to be sure they were reading each question, a “low incidence item” question and a “speeder trap” to ensure the final sample included only high-quality surveys.
The survey sample sought to mirror the demographic composition of Arizona, the geographic area of interest. We employed weighting to compensate for sample designs and patterns of non-response that might bias results. To handle missing data among some of the self-reported demographic variables for purposes of weighting only, we employed a technique termed “hot decking,” which replaces the missing values of a respondent randomly with another similar respondent without missing data. These are further determined by variables predictive of non-response that are present in the entire file (see technical appendix for more details)(24, 25). Weighting was accomplished using SPSS INC RAKE, an SPSS extension module that simultaneously balances the distributions of all variables. The weighting parameters were sex, age, education, race/ethnicity, region of Arizona, civic engagement, marital status, and household income, derived from the September 2019 Current Population Survey Volunteering and Civic Life Supplement (civic engagement) or the 2019 American Community Survey (sex, age, education, race/ethnicity, region of Arizona, marital status, and household income). Weights were trimmed at the 2nd and 98th percentiles to prevent individual interviews from having too much influence on the final results.
Statistical Analysis
All data analyses were performed in SPSS version 27. We calculated descriptive statistics for survey questions of interest in the whole group, then by ethnicity and by age group for questions of interest. We then compared the distribution of answers between Hispanic and non-Hispanic White respondents at all ages with additional comparisons made between the ethnic groups in only those at younger ages, between 35-54 years-old, using chi-squared tests. We also examined differences in responses between all participants by age, comparing younger respondents (age 35-54) to older respondents (55 and over), again using chi-squared tests.
Results
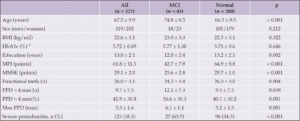
A total of 1,303 persons aged 35 and older, of whom 332 (25.5%) were Hispanic and 842 (64.6%) non-Hispanic White, responded to the survey between July 13th and August 2nd, 2021. 63.3% of respondents were female. The characteristics of the survey population are shown in Table 2.
Attitudes in Hispanic Compared to Non-Hispanic White Respondents
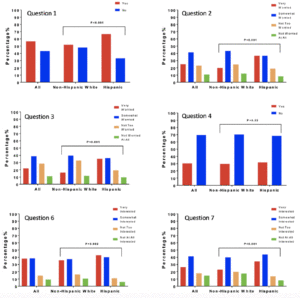
Hispanic respondents were more likely to report having a close friend or relative who had been diagnosed with AD or dementia compared to non-Hispanic White respondents (66.8% vs 51.9%, X2 (1, N=1226) = 24.1, p<0.001). Hispanic respondents also reported a higher degree of concern about a close relative (73.1% vs 63.2% being “very” or “somewhat worried”, X2 (3, N=1226) = 40.3, p<0.001) as well as themselves (71.3% vs 55.6% being “very” or “somewhat worried”, X2 (3, N=1226) = 63.1, p<0.001) being diagnosed with AD or dementia in the future compared to Non-Hispanic White respondents. The concern about a future diagnosis being more prominent among Hispanic respondents remained evident when limiting analyses to younger individuals 35-54 years old (75.4% vs 69.8% “very” or “somewhat interested,” X2 (3, N=500) = 16.5, p<0.001).
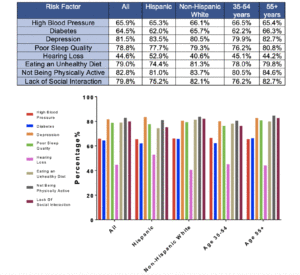
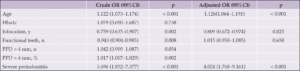
When asked, “are you aware of any risk factors that increase the risk for developing AD or dementia later in life?” (Table 1, Question 4), 30.7% of respondents reported being aware of such factors and there was no significant difference between non-Hispanic White and Hispanic respondents (31.6% vs 29.8%, X2 (1, N=1226) = 0.41, p=0.52). When the general question about risk factors was followed up by asking whether a specific item such as diabetes or physical inactivity are risk factors for dementia (Table 1, Question 5), the percentage of persons reporting awareness of individual risk factors increased significantly, generally doubling or more with the exception of hearing loss. Hearing loss was the risk factor with the least awareness, with Hispanic respondents being more likely to be aware of this risk factor. There were no statistically significant differences between the two groups in awareness of any other risk factors. Figure 1 summarizes the percentages of persons reporting awareness of individual risk factors for AD/ADRD in the overall group, as well as by ethnicity and age.
We next asked about interest in a dementia prevention program (Table 1, Question 6). 76.4% of respondents reported being “very” or “somewhat interested” in such a program. Interest was significantly higher in Hispanic compared to non-Hispanic White respondents (83.0% vs 73.3% “very” or “somewhat interested,” X2 (3, N=1226) = 14.8, p=0.002). Reponses of the entire sample and comparisons between Hispanic and non-Hispanic White respondents for this and other survey questions are summarized in Figure 2. Hispanic male respondents were significantly more likely than non-Hispanic White male respondents to have interest in a dementia prevention program (86.2% vs. 71.9% being “very” or “somewhat interested,” X2 (3, N=444) = 15.2, p=0.002), while Hispanic female respondents were numerically more interested in a program compared to non-Hispanic White females, the difference in interest was not significant (80.7% vs. 74.1%, X2 (3, N=778) = 3.9, p=0.269). Stratifying by income level did not significantly alter results, Hispanic respondents expressed greater interest in a dementia prevention program in both the low (<$50,000/year) and greater (>$50,000/year) income groups (83.0% vs. 71.9% being “somewhat” or “very interested” for the income <$50,000 group, X2 (3, N=450) = 8.0, p=0.046, and 84.7% vs. 71.4% X2 (3, N=590) = 13.2, p=0.004 for the income >$50,000 group). Hispanic participants remained more interested in a prevention program regardless of education level (X2 (3, N=40) = 12.9, p=0.005 for those with a high school degree or less, X2 (3, N=1186) = 12.6, p=0.005) for those with more than a high school degree).
Next, we asked about interest in participation in a clinical trial aimed at preventing or delaying Alzheimer’s disease or dementia (Table 1, Question 7). Hispanic respondents had greater interest in participating in AD/ADRD prevention research (78.1% Hispanic vs 62.8% Non-Hispanic White were “very” or “somewhat interested”, X2 (3, N=1226) = 35.1, p<0.001). This finding was consistent when limiting analyses to younger respondents, age 35-54 (80.7% vs. 68.4%, X2 (3, N=500) = 15.6, p=0.001) as well as both only male (82.4% vs. 61.8%, X2 (3, N=444) = 30.3, p<0.001) and only female respondents (74.9% vs. 63.1%, X2 (3, N=778) = 10.4, p=0.015).
Attitudes in Younger Compared to Older Respondents
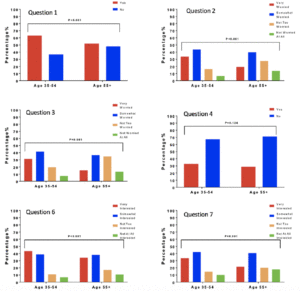
Younger participants (age 35-54) were more worried about themselves being diagnosed with AD or another dementia in the future (72.4% vs. 51.5%, X2 (3, N=1302) = 74.9, p <0.001) compared to older respondents (age 55 and older). This remained the case when comparing younger with older Hispanic respondents, with 74.8% of the younger group versus 61.1% of the older group reporting that they were either “very” or “somewhat worried” (X2 (3, N=383) = 9.9, p=0.019). Comparisons between younger and older respondents are shown in Figure 3.
There were no significant differences between younger and older persons in awareness of risk factors that increase the risk of developing AD/ADRD later in life (33.4% vs. 28.8%, X2 (1, N=1302) = 3.1, p =0.076) though again surprisingly the trend was for younger persons to be more aware of risk factors. Interest in participating in a program targeting dementia prevention was greater in the younger population. 83.0% of younger participants were either “very interested” or “somewhat interested” in participating in a dementia prevention program versus 72.1% of older participants (X2 (3, N=1302) = 20.0, p<0.001). Younger respondents also reported significantly greater interest in being referred for a drug or clinical trial to prevent, delay, or treat AD or dementia if one were available (75.0% vs. 61.7% being “somewhat” or “very interested,” X2 (3, N=1302) = 33.6, p<0.001).
Discussion
This study provides insight into the degree of awareness of risk factors for dementia, interest in participating in a prevention program, and respective differences between Hispanic and non-Hispanic White persons as well as younger and older persons in Arizona. We found that a significant majority of respondents reported not knowing about any risk factors for dementia, with no difference between Hispanic and non-Hispanic White participants. While less than 1/3 of respondents reported being aware of risk factors, about 3/4 were interested in dementia prevention programs, indicating that an important disconnect exists between the desire for addressing modifiable risk factors to lower dementia risk and the ability to accomplish this goal.
Next, we found that Hispanic respondents had a higher degree of interest in a dementia prevention program than non-Hispanic White respondents. This may follow from the fact that Hispanic respondents were also more likely to know someone with dementia and be more worried both about a relative and themselves developing dementia in the future. This interest, paired with the fact that Hispanic persons have more prevalent and poorly addressed modifiable risk factors, suggests dementia prevention programs have the potential to provide greater benefit to Hispanic communities, which could narrow outcome disparities. It is important to point out that, at a minimum, access to such clinics would need to be equitable, if not somewhat designed, tailored, and with recruitment skewed toward underrepresented communities. While interest in a dementia program is encouraging, interest alone does nothing to address existing barriers to implementation (28). Increasing healthcare resources, both in terms of education and delivery, are required to meet the existing interest in underrepresented communities to make an impact. Increasing investment in preventative and early intervention programs for common but addressable risk factors for dementia may be one such strategy.
This study also found that Hispanic respondents were more interested in participating in a prevention clinical trial than non-Hispanic White respondents. This contrasts with historical underrepresentation of Hispanic participants in AD/ADRD clinical trials and suggests that the problem is not a fear of, or a lack of interest in participation, but rather efforts of studies and trials to reach and provide access to diverse participants. Congruent with our finding is a national survey that found no differences in willingness to participate in a prevention trial among socioeconomic groups (26) and the recent report from the United States Study to Protect Brain Health Through Lifestyle Intervention to Reduce Risk (US POINTER), which successfully enrolled 30.8% non-White participants (27).
Most clinical programs and trials are focused on older individuals. Here, we found interest in a dementia prevention program was higher among younger individuals ages 35-54 compared to those age 55 and older suggesting that inclusion of younger individuals in prevention clinics is feasible. There may be several reasons for this finding. Cognitive decline and dementia were considered to be a normal part of aging in previous decades. Concerted efforts have sought to dispel this notion, and younger individuals may acknowledge the fact that dementia is not a normal part of aging more than older individuals. They may also be aware of interventions to lower the risk of this outcome, whereas older individuals might view cognitive decline and dementia as an inevitable part of aging. Another possible explanation is in the changing attitudes regarding overall health among generations, with prioritization of “wellness,” (defined as “the active pursuit of activities, choices, and lifestyles that lead to a state of holistic health” (28)) being more prominent in younger generations. For example, with respect to diet, millennials (those born between 1981 and 1996) are much more likely (80% vs 64%) to prioritize health and wellness when making food choices as compared to baby boomers (those born between 1946 and 1964) (29). A similar phenomenon may be occurring in this study, with younger persons having a greater interest in proactively maintaining their health and wellness through lifestyle choices. Finally, there may be some degree of selection bias in the sample, with younger persons being more likely to participate in the survey if they had prior experience and knowledge of dementia and interest in ways to lower future risk.
Limitations
Study limitations include a reliance on self-reported data on education, income, and other demographic data. The study was conducted online and thus likely excluded some persons with no or little access to reliable internet. The sample was predominately female (63.3%). Next, respondents were not paid to complete the survey, and there may be some degree of selection bias of persons who have an interest in dementia prevention and modifiable risk factors as being more likely to offer their time and effort to complete the survey. This selection bias may have been introduced to a higher degree in younger persons who would presumably be more occupied with their careers and younger families, requiring a greater interest in the topic to motivate participation and completion. These limitations may have rendered the survey population less representative of the general population, reducing generalizability, and potentially skewing results to some degree. The sample was also intentionally representative of Arizona residents, and thus may not be generalizable beyond this geographic area. These limitations could be mitigated in future research by increasing geographic reach, obtaining more complete demographic information, offering monetary compensation for participation, and including non-web-based/electronic formats to promote participation among those with limited internet access.
Conclusions
Among a representative sample of Arizona residents, knowledge of modifiable risk factors for dementia was low, contrasting with the high degree of interest in a dementia prevention program addressing these risk factors. Interest in a clinic was higher among Hispanic compared to non-Hispanic White and younger compared to older respondents. Implementation of a cognitive health clinic aimed at dementia prevention inclusive of young and diverse participants is feasible in Arizona. Provided that access is equitable among ethnic groups, a dementia prevention program addressing modifiable risk factors may be one way to narrow AD/ADRD outcome disparities.
Funding: Women Investing in Science and Health of the Banner Health Foundation sponsored this study. The sponsors had no role in the design and conduct of the study; in the collection, analysis, and interpretation of data; in the preparation of the manuscript; or in the review or approval of the manuscript.
Conflict of Interest Disclosure: Harshita Talkad, Yinghua Chen, and Adam Bress declare no conflicts of interest. Jessica Langbaum has received grant support from the NIH/NIA; State of Arizona, Arizona Alzheimer’s Consortium; and Eli Lilly; and has received consulting fees from Alector, Biogen, and Denovo Biopharma. Pierre Tariot has received grant support from the NIA and consulting fees from AbbVie, AC Immune, Acadia, Athira, Biogen, BioXcel, Bristol Myers Squibb, Cognition Therapeutics, Corium, Cortexyme, CuraSen, Eisai, Genentech, Immunobrain, Janssen, Lundbeck, MapLight, Merck & Co., Novartis, Novo Nordisk, Otsuka & Astex, Roche, Syneos, and T3D Therapeutics. He has received honoraria from Arizona Osteopathic Medical Association, Arbor Scientia, Clinical Care Operations, Health & Wellness Partners, Indiana University, Merck, Miller Medical Communications, River West Meeting Associates, Tucson Osteopathic Medical Foundation, and University of Cincinnati. He is a contributor to U.S. Patent #11/632, 747, “Biomarkers of Neurodegenerative Disease” and participates on a Data Safety Monitoring Board or Advisory Board for AbbVie, AC Immune, Acadia, Athira, Corium, Cortexyme, Eisai, Genentech, ImmunoBrain, Merck, Novo Nordisk, and Syneos Health. Jeremy Pruzin received support for this manuscript from Women Investing in Science and Health and the Banner Health Foundation. He has grant support from the NIA as a co-investigator, received honoraria from the American Academy of Neurology and consulting fees for an Eisai advisory board.
Ethical standards: Survey participants consented to participate in the survey through opting in to the web-based panel. All data was de-identified and remained so once collected. The study was determined to be Institutional Review Board exempt (Health and Human Resources §46.104 Exemption 3).
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
1. Jack CR, Jr., Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12(2):207-16.
2. Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413-46.
3. Kramer AF, Hahn S, Cohen NJ, Banich MT, McAuley E, Harrison CR, et al. Ageing, fitness and neurocognitive function. Nature. 1999;400(6743):418-9.
4. Rabin JS, Klein H, Kirn DR, Schultz AP, Yang HS, Hampton O, et al. Associations of Physical Activity and β-Amyloid With Longitudinal Cognition and Neurodegeneration in Clinically Normal Older Adults. JAMA Neurol. 2019;76(10):1203-10.
5. Yoon M, Yang PS, Jin MN, Yu HT, Kim TH, Jang E, et al. Association of Physical Activity Level With Risk of Dementia in a Nationwide Cohort in Korea. JAMA Netw Open. 2021;4(12):e2138526.
6. Buchman AS, Boyle PA, Yu L, Shah RC, Wilson RS, Bennett DA. Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology. 2012;78(17):1323-9.
7. Diem SJ, Blackwell TL, Stone KL, Yaffe K, Tranah G, Cauley JA, et al. Measures of Sleep-Wake Patterns and Risk of Mild Cognitive Impairment or Dementia in Older Women. Am J Geriatr Psychiatry. 2016;24(3):248-58.
8. Sabia S, Fayosse A, Dumurgier J, van Hees VT, Paquet C, Sommerlad A, et al. Association of sleep duration in middle and old age with incidence of dementia. Nat Commun. 2021;12(1):2289.
9. Penninkilampi R, Casey AN, Singh MF, Brodaty H. The Association between Social Engagement, Loneliness, and Risk of Dementia: A Systematic Review and Meta-Analysis. J Alzheimers Dis. 2018;66(4):1619-33.
10. Morris MC, Tangney CC, Wang Y, Sacks FM, Bennett DA, Aggarwal NT. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimers Dement. 2015;11(9):1007-14.
11. Barnes LL, Dhana K, Liu X, Carey VJ, Ventrelle J, Johnson K, et al. Trial of the MIND Diet for Prevention of Cognitive Decline in Older Persons. N Engl J Med. 2023;389(7):602-11.
12. Durazzo TC, Mattsson N, Weiner MW. Smoking and increased Alzheimer’s disease risk: a review of potential mechanisms. Alzheimers Dement. 2014;10(3 Suppl):S122-45.
13. Lennon MJ, Makkar SR, Crawford JD, Sachdev PS. Midlife Hypertension and Alzheimer’s Disease: A Systematic Review and Meta-Analysis. J Alzheimers Dis. 2019;71(1):307-16.
14. Gottesman RF, Albert MS, Alonso A, Coker LH, Coresh J, Davis SM, et al. Associations Between Midlife Vascular Risk Factors and 25-Year Incident Dementia in the Atherosclerosis Risk in Communities (ARIC) Cohort. JAMA Neurol. 2017;74(10):1246-54.
15. Barbiellini Amidei C, Fayosse A, Dumurgier J, Machado-Fragua MD, Tabak AG, van Sloten T, et al. Association Between Age at Diabetes Onset and Subsequent Risk of Dementia. Jama. 2021;325(16):1640-9.
16. 2023 Alzheimer’s disease facts and figures. Alzheimers Dement. 2023;19(4):1598-695.
17. Wilkins CH, Windon CC, Dilworth-Anderson P, Romanoff J, Gatsonis C, Hanna L, et al. Racial and Ethnic Differences in Amyloid PET Positivity in Individuals With Mild Cognitive Impairment or Dementia: A Secondary Analysis of the Imaging Dementia-Evidence for Amyloid Scanning (IDEAS) Cohort Study. JAMA Neurol. 2022;79(11):1139-47.
18. Barnes LL, Leurgans S, Aggarwal NT, Shah RC, Arvanitakis Z, James BD, et al. Mixed pathology is more likely in black than white decedents with Alzheimer dementia. Neurology. 2015;85(6):528-34.
19. McGrath ER, Beiser AS, O’Donnell A, Himali JJ, Pase MP, Satizabal CL, et al. Determining Vascular Risk Factors for Dementia and Dementia Risk Prediction Across Mid- to Later-Life: The Framingham Heart Study. Neurology. 2022;99(2):e142-53.
20. Hawes AM, Smith GS, McGinty E, Bell C, Bower K, LaVeist TA, et al. Disentangling Race, Poverty, and Place in Disparities in Physical Activity. Int J Environ Res Public Health. 2019;16(7).
21. McCullough ML, Chantaprasopsuk S, Islami F, Rees-Punia E, Um CY, Wang Y, et al. Association of Socioeconomic and Geographic Factors With Diet Quality in US Adults. JAMA Netw Open. 2022;5(6):e2216406.
22. Seifan A, Isaacson R. The Alzheimer’s Prevention Clinic at Weill Cornell Medical College / New York – Presbyterian Hospital: Risk Stratification and Personalized Early Intervention. J Prev Alzheimers Dis. 2015;2(4):254-66.
23. Isaacson RS, Hristov H, Saif N, Hackett K, Hendrix S, Melendez J, et al. Individualized clinical management of patients at risk for Alzheimer’s dementia. Alzheimers Dement. 2019;15(12):1588-602.
24. Andridge RR, Little RJ. A Review of Hot Deck Imputation for Survey Non-response. Int Stat Rev. 2010;78(1):40-64.
25. Myers T. Goodbye, Listwise Deletion: Presenting Hot Deck Imputation as an Easy and Effective Tool for Handling Missing Data. Communication Methods and Measures. 2011;5:297-310.
26. Cox CG, Davis MA, Grill JD, Roberts JS. US Adults’ Likelihood to Participate in Dementia Prevention Drug Trials: Results from the National Poll on Healthy Aging. J Prev Alzheimers Dis. 2023;10(1):34-40.
27. Whitmer R. U.S. Study to Protect Brain Health Through Lifestyle Intervention to Reduce Risk (U.S. POINTER): Baseline Characteristics of a Trial Cohort Enriched for Risk of Cognitive Decline2023. Available from: https://alz-journals.onlinelibrary.wiley.com/doi/10.1002/alz.082801.
28. Institue GW. 2020 Compendium: Resetting the World with Wellness 2020 [Available from: https://globalwellnessinstitute.org/industry-research/resetting-the-world-with-wellness/.
29. Council IFI. 2023 Food & Health Survey 2023 [Available from: https://foodinsight.org/2023-food-health-survey/.
© The Authors 2024