A COMPARATIVE, DOUBLE-BLIND, PLACEBO CONTROLLED ACUTE CLINICAL STUDY
T. Reyes-Izquierdo1, M.J. Phelan2, R. Keller3, C. Shu1, R. Argumedo1, Z. Pietrzkowski1
1. FutureCeuticals Inc., Applied BioClinical Laboratory, 16259 Laguna Canyon Rd, Irvine, CA, USA 92618; 2. Department of Statistics, School of Information and Computer Science. 2026 Donald Bren Hall, University of California at Irvine, Irvine, CA, 92697; 3. NutraClinical Inc., 16259 Laguna Canyon Rd, Irvine, CA USA92618
Corresponding Author: Tania Reyes-Izquierdo, Ph.D. 16259 Laguna Canyon Rd, Irvine CA, 92618 USA, Phone +1 949 502 4496, Fax +1 949 502 4987, Email: tania@abclinicaldiscovery.com
Corrigendum
Disclosure Statement: The present study was funded by VDF FutureCeuticals, Inc (Momence IL, USA). At the time of submission, T. Reyes-Izquierdo, R. Argumedo, amd Z. Pietrzkowski were employed by VDF FutureCeuticals, Inc.
Abstract: The following text replaces the Abstract in its entirety:
Abstract: Purpose: To compare and evaluate the effects of treatment with a blend of glucosamine, chondroitin sulfate and calcium fructoborate as compared to a placebo, on joint discomfort. Methods: Individuals with self-reported knee discomfort were randomized and blinded to treatment with a combo containing glucosamine and chondroitin sulfate or glucosamine, chondroitin sulfate and calcium fructoborate. Both groups were compared to placebo. Symptoms of discomfort and joint function were assessed using the Western Ontario and McMaster Universities Arthritis Index (WOMAC) and the McGill Pain Questionnaire (MPQ) before treatment and after 7 and 14 days of treatment. Results: For these three arms, ninety-six individuals were selected and were randomly assigned into groups of 32, each containing an equal number of males and females. Treatment with glucosamine combined with chondroitin sulfate and CFB resulted in a statistically significant 24% reduction of mean WOMAC score and a 25% reduction of mean McGill index at day 14 over baseline (p-value = 0.0006 and p-value < 0.0001, respectively). Conclusions: Results showed that short-term use of CFB in combination with chondroitin sulfate and glucosamine was effective in reducing knee discomfort and improving the physical mobility of the joints.
Materials & Methods: Study Description
The following text provides additional information to supplement the Material and Methods: Study Description section:
The study was comprised of four arms that included one placebo group and three treatment groups, as follows:
(1) Calcium Fructoborate (“CFB”)
(2) Glucosamine and Chondroitin (“GC”) and CFB
(3) GC
(4) Placebo
This study tested two hypotheses: (1) that CFB would be effective for short term treatment of joint pain and (2) that a combination of CFB and GC would be effective for short term treatment of joint pain. Results relating to CFB alone were reported in Pietrzkowski et al., “Short-term efficacy of calcium fructoborate on subjects with knee discomfort: a comparative, double-blind, placebo-controlled clinical study.” Clinical interventions in aging 9 (2014): 895. Results relating to a combination of CFB and GC are reported herein.
Materials & Methods: Experimental Groups
The following text provides additional information to supplement the Material & Methods: Experimental Groups section:
Subjects in the placebo group and CFB (alone) group were instructed to take one capsule twice per day. Subjects in the GC and CFB group (i.e., TR1) and the GC (alone) (i.e., TR2) group were instructed to take two capsules twice per day.
Results
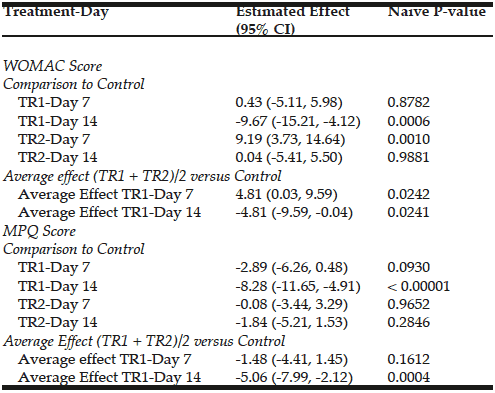
The WOMAC p-value was correctly reported as 0.0006 in Table 3. However, the Results section of the article contained a typographical error, incorrectly referring to the same WOMAC p-value as “0.006.”
Abstract
Purpose: To compare and evaluate the effects of treatment with a blend of glucosamine and chondroitin sulfate, or a blend of glucosamine, chondroitin sulfate and calcium fructoborate as compared to a placebo, on joint discomfort. Methods: Individuals with self-reported knee discomfort were randomized and blinded to treatment with a combo containing glucosamine and chondroitin sulfate or glucosamine, chondroitin sulfate and calcium fructoborate. Both groups were compared to placebo. Symptoms of discomfort and joint function were assessed using the Western Ontario and McMaster Universities Arthritis Index (WOMAC) and the McGill Pain Questionnaire (MPQ) before treatment and after 7 and 14 days of treatment. Results: Ninety individuals were selected for this study and were randomly assigned in groups of 30 containing 15 male and 15 female participants to each of three treatment conditions. Treatment with glucosamine combined with chondroitin sulfate and CFB resulted in a statistically significant 24% reduction of mean WOMAC score and a 25% reduction of mean McGill index at day 14 over baseline (p-value = 0.0006 and p-value < 0.0001, respectively). Treatment with placebo or with glucosamine and chondroitin material did not result in significant improvement of the conditions. Conclusions: Results showed that short-term treatment with glucosamine and chondroitin could be efficacious only if used in combination with CFB.
Key words: Calcium fructoborate, glucosamine, chondroitin sulfate, WOMAC, McGill, joint discomfort.
Introduction
Chronic knee discomfort is a common phenomenon among population of older age (1). Many circumstances can cause or contribute to it (2). Individuals experiencing knee discomfort often report long-term distress, swelling, or sensitivity in one or both knees. Conventional management of knee discomfort mainly focuses on relief of symptoms using analgesics and non-steroidal anti-inflammatory drugs (NSAIDs) (3, 4). Some dietary supplements have reported to show some potency to reduce symptoms associated with joint discomfort (5-10).
Chondroitin sulfate is an important structural component of cartilage. Chemically, it is a sulfated glycosaminoglycan composed of N-acetyl-galactosamine and glucuronic acid (11). Over the years, this material has been used as a dietary supplement to treat symptoms of joint discomfort. This material is bioavailable at the level 15-24% of orally administered dose (12-15). It has been suggested that ingested chondroitin sulfate may show anti-inflammatory activity, inhibit proteolytic activity, stimulate the synthesis of proteoglycans and hyaluronic acid and reduce catabolic activity of chondrocytes (16). This material has been used to reduce deficiency and degradation of endogenous chondroitin sulfate in the body, to modulate IL-1β and Nf-kB in chondrocytes, activities that have been suggested may improve OA conditions if chondroitin is used over an extended time. (15, 17-19) However, clinical potency of chondroitin sulfate remains a subject of open discussion due to mixed efficacy results (15, 20-22). Recent studies have addressed the efficacy of treatment with chondroitin and have concluded that some positive results may be observed after long-term supplementation (23-25).
Another dietary glycan that has been suggested to have beneficial effects on joint discomfort is glucosamine sulfate. Glucosamine sulfate is an amino sugar and a prominent precursor in the biochemical synthesis of glycosylated proteins and lipids. Glucosamine sulfate (“glucosamine”) has been marketed to relieve symptoms of osteoarthritis, which involves the age-dependent erosion of articular cartilage (25, 26).
Glucosamine is marketed to support the structure and function of joints in people suffering from joint discomfort. Commonly sold forms of glucosamine are glucosamine sulfate, glucosamine hydrochloride, and N-Acetyl Glucosamine. Although there is no evidence to suggest that glucosamine sulfate offers advantages over medications estrace is a form of estrogen – the female hormone necessary for many processes in the body. generic estrace is used to treat symptoms of glucosamine hydrochloride, the latest does not need to be stabilized with salt and offers a more concentrated form of glucosamine. Given these facts, the product of choice for consumers should be Glucosamine hydrochloride (27). The use of glucosamine for management of OA conditions has been studied and reported in a manner similar to chondroitin sulfate; however, clinical potency of treatment of OA conditions with glucosamine remains unclear; despite previously performed clinical studies (28) A mixture of these dietary supplements (glucosamine and chondroitin) has been used to help delay or reverse the loss of cartilage (29, 30).
Calcium fructoborate (CFB) is a natural borate complex first described by Miljkovic (31) CFB has been tested in clinical studies to verify its potency to reduce joint discomfort symptoms and certain inflammatory markers (32, 33). Previous studies have shown that treatment with CFB resulted in statistically significant reduction of blood C-Reactive Protein (CRP) levels in subjects with angina pectoris (34, 35) and in subjects with increased risk of cardiovascular conditions (36). CFB is a patented, nature-identical compound produced according to a proprietary process described by Miljkovic (US Patent #5,962,049) (31). Chemical structure and identity of CFB has been previously described (32). Based upon these previous observations, we have designed this short term pilot clinical trial wherein CFB has been combined with glucosamine and chondroitin sulfate in order to observe possible effects on knee discomfort.
Materials and Methods
Materials
CFB was provided by VDF FutureCeuticals, Inc., (Momence, IL, USA). Glucosamine hydrochloride, chondroitin sulfate, silica oxide and fructose were from Sigma-Aldrich (St Louis, MO, USA),
Inclusion criteria for study subjects: Age range: >35 and<65 years; BMI: >21 and <30; no visible evidence of having a cold or other infections; non-diabetic; free of allergies; McGill Score >35 but less than 60.
Exclusion criteria
Age range: <35 and >65 years; BMI: <21 or >30 (kg/ m2); diabetes; subjects who were pregnant, nursing, or planning to get pregnant; subjects currently enrolled in another study; subjects with cardiovascular diseases; subjects taking medications for pain or NSAIDs, subjects taking supplements or vitamin D within two weeks of this trial.
Consent
This study was conducted according to the guidelines put forth in the Declaration of Helsinki and all procedures involving human subjects were approved by the Institutional Review Board at Vita Clinical S.A. (Avenida Circunvalacion Norte #135, Guadalajara, JAL, Mexico 44270) (IRB Number: ABC-NCI-13-08-FRXB). All study subjects were generally healthy and had not used any type of medication or supplement for a period of 15 days prior to the start of the study. Study was performed by NutraClinical Inc. (San Diego, USA) according to the study protocol designed by VDF FutureCeuticals, Inc./ Applied Bioclinical Lab (Irvine, CA, USA).
Study description
After protocol had been approved by the Institutional Review Board, male and female subjects between 35-65 years of age were prescreened according to the inclusion and exclusion criteria. Subjects were recruited through advertisement in local papers. Distribution of the treatments and data collection was performed by NCI (NutraClinical Inc. 16259 Laguna Canyon Rd., Irvine, CA 92618). A total of 96 subjects were selected to participate in the study. All admitted participants had McGill scores between 35 and 60 (per inclusion criteria) and all had given written consent. Participants were randomly assigned into three (3) groups of 32 subjects. Each group contained an equal number of males and females. Each group was subjected to one of three treatment conditions. On Day 1 (baseline) medical history and physical examinations were performed on all subjects. Blood collections were performed on days 1 (baseline), 7 and 14 and always under fasting conditions (12h). On day 1, all subjects received their test products and were instructed to take the first dose immediately after blood collection. All bottles containing the tested materials and all capsules were similar in appearance. McGill and WOMAC Questionnaires were administered at baseline and at 7 and 14 days.
Experimental Groups
Placebo contained 80mg of fructose and 15mg of silica oxide per capsule. Subjects in the placebo group were instructed to take one (1) capsule twice per day before meals. Treatment 1 (TR1) capsules contained a mixture of 375 mg glucosamine, 100 mg chondroitin sulfate and 55 mg CFB. Treatment 2 (TR2) capsules contained a mixture of only 375mg of Glucosamine and 100mg of Chondroitin sulfate. Subjects in TR1 and TR2 experimental groups were advised to take two capsules twice per day before meals. All subjects were instructed to take the capsules before breakfast and lunch and minimum fifteen minutes prior to eating.
Western Ontario and McMaster Universities Arthritis Index. The Western Ontario and McMaster Universities Arthritis Index (WOMAC) is a widely used questionnaire used to calculate physical function of joints (37). The WOMAC consists of 24 items divided into 3 subscales: pain (5 questions; scores range from 0 to 20), stiffness (2 items; scores range from 0 to 8), and functional limitations (17 items; scores range from 0 to 68). Total scores range from 0 (best) to 96 (worst). The WOMAC index was administered on day 1, day 7 and day 14 of treatment.
McGill Pain Questionnaire. The McGill Pain Questionnaire (MPQ) is a multidimensional pain questionnaire used to quantify the quality and intensity of pain (38, 39). The questionnaire was designed to provide quantitative measures of clinical pain that can be treated statistically. The scale contains 4 subscales consisting of 78 words that participants use to describe feelings of pain. On the first category, subjects have to select a word that “describes” the pain (like “quivering”, “pounding”). The second category includes the pain components (“tiring”, “suffocating”). The third category is the evaluation of the pain (from “no pain” to “excruciating”). The last part includes a miscellaneous description (“spreading”, “torturing”, “miserable”). After completing the questionnaire, users will have selected seven words that best describe their pain. Each chosen word has an associated numerical value, giving a total score ranging from 0 (no pain) to 78 (severe pain). The McGill pain questionnaire was administered on day 1 (pre-treatment) and after 7 and 14 days of treatment.
Blood Collection
Blood was collected at baseline prior to treatment. For each participant, two 9 mL blood samples were drawn from an antecubital vein in anticoagulant-free (dry tubes) (BD Vacutainer Franklin Lakes, NJ, USA). Blood again was drawn at Day 7 and Day 14 of the treatment, and always under fasted conditions.
Statistical Methods
In order to address the a priori hypothesis that treatment would improve mean reported discomfort in study subjects with self-reported discomfort in knee joint, the primary analysis tested the effect of treatment on the mean 7-day and 14-day change from baseline in WOMAC score (Western Ontario and McMaster Universities Arthritis Index) and McGill score (pain index of McGill University). A repeated measures analysis of variance (ANOVA) (40) was used to estimate treatment effects on within-subject changes in mean WOMAC and McGill scores over the 7- and 14-day period. Specifically, each score was regressed on an indicator of treatment group, post-treatment day, and the treatment-day interaction. In this case, a test of the coefficients for the treatment-day interaction equaling zero is equivalent to a test of the treatment effect at day 7 and day 14. Both the WOMAC and McGill scores were analyzed using this approach.
Results
Age and BMI characteristics of the study population are presented in Table 1. The average age of the 92 study participants was 49.2 years. The groups were comparable with respect to BMI (26.6 kg/m2 for all 92 women’s health. body- building, hypnotherapy, herbals, male enhancement. study subjects). Fifty percent of subjects in each treatment group were male. Two participants on the control group and 2 on TR1 did not follow compliance and were dropped from the study. A total of 92 participants remained on study through visits 1, 2 and 3. Numerical summaries of WOMAC and MPQ scores are listed in Table 2. At baseline, range of WOMAC values was 41.6-53.5 and range of McGill score values was 49.2-51.5. It is noticeable that the average value of WOMAC in TR2 was lower comparing to control and TR1 at baseline. However, MPQ score average values were in the same range in all experimental groups. The analysis of treatment effects was based on mean within subject change from baseline.

Table 1: Characteristics of study subjects as presented by an average values (mean+/-SEM) at Day 1 (baseline)
Abbreviations: BMI, body mass index; WOMAC, Western Ontario and McMaster Universities Arthritis index; McGill, McGill Pain Questionnaire; SE, Standard Error of the Mean
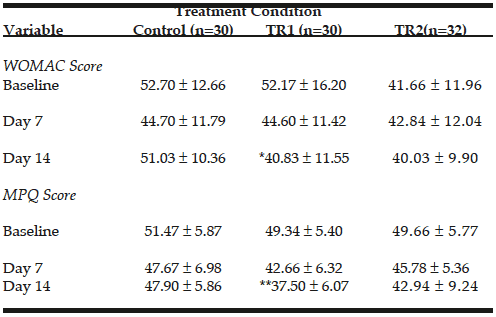
Table 2: Numerical Summaries of WOMAC and MPQ score by Treatment and Day. Reporting AVE ± SD. WOMAC and MPQ values were determined at baseline and on day 7 and 14
Abbreviations: WOMAC, Western Ontario and McMaster Universities Arthritis Index; MPQ, McGill Pain Questionnaire; AVE, average; SD, standard deviation. *Significant difference from baseline P-value=0.006. **Significant difference from baseline P-value >0.00001
Table 3 shows the estimated effects of active treatment versus placebo at 7 and 14 days follow up. In each case the mean within-subject change from baseline for active compounds is compared to the mean within-subject change for the placebo. A negative estimate indicates that treatment was involved in greater reductions in reported discomfort. The within-subject change in mean WOMAC score over 14 days was estimated to be 9.67 points lower on TR1 (glucosamine, chondroitin sulfate plus CFB) when compared to control (Estimate effect = -9.67, P-Value = 0.006). The within-subject change in mean McGill score over 14 days was estimated to be 8.28 points greater on TR1 when compared to control (Estimated effect = -8.28, P-Value < 0.00001). TR2 (glucosamine and chondroitin, alone) appeared to have an effect on WOMAC scores at day 7, but it is in the wrong direction. This is interpreted to be an artifact associated with the lower mean WOMAC score in group TR2 at baseline. The apparent effect vanished at day 14, and no effect of TR2 is reported on McGill scores throughout the follow-up period. Generally, for both the WOMAC and McGill score, TR1 was more effective than TR 2 at reducing knee discomfort over the two week period.
Blood chemistry analysis performed at Days 1, 7 and Day 14 did not indicate any significant changes in blood levels of key electrolytes, enzymes, lipids and glucose. All subjects completed this trial without any indications of unusual effects.
Discussion
Chronic knee discomfort is a condition that significantly affects the quality of life due to its impact on physical movements. Conventional treatment for joint discomfort involves the use of NSAIDs and dietary supplements such as glucosamine and chondroitin supplements. Since NSAIDs are associated with potential side effects(3, 4, 41, 42); the rationale behind the use of dietary supplements is to improve chronic joint discomfort while reducing the need for NSAIDS. Chondroitin sulfate and glucosamine supplements have been commonly used as dietary supplements to reduce and/or slow down cartilage damage in subjects with joint discomfort (43-47).
Calcium fructoborate (CFB) is a natural plant mineral borate complex produced by a patented process first described by Miljkovic (Miljkovic et al., US Patent #5,962,049) (31). Of the existing boron and borate supplements available, CFB might be the most researched and might offer the most potential for human health (31, 48). CFB, a potential anti-inflammatory agent (35, 49, 50) with the ability to modulate key markers associated with inflammation-related conditions, such as osteoarthritis (32, 50, 51), has been recently reported to subjectively improve feelings of flexibility, comfort, and quality of life in a period of only 14 days (33). For this study, we chose a combination of CFB, along with glucosamine hydrochloride and chondroitin sulfate to assess any synergistic effect. The objective of this study was to investigate any possible short-term benefits in decreasing discomfort and improving physical mobility in participants during a short period of time (only 14 days). We also observed the effects of using a combination of chondroitin sulfate and glucosamine alone. Even though the recruited participants had not been previously diagnosed, the criterion of selection was based on the McGill Pain Questionnaire, which is not only used to evaluate and monitor pain, but also to determine the effectiveness of any intervention. Discomfort and physical function of the joints were recorded for baseline, Day 7 and Day 14 of the study period. The data tables from the study show that at the start of the study all participants had similar discomfort and physical function scores (Table 2). Results presented in Table 3 showed that TR1 (a combination of CFB, chondroitin and glucosamine) significantly reduced mean WOMAC and McGill scores when compared to placebo. In contrast, TR2 using only chondroitin sulfate and glucosamine had no effect under these experimental conditions. Although our study showed that the combination of all 3 nutrients can result in significant rather rapid improvement in knee discomfort and improved mobility, further investigation is justified and can yield additional insight into these materials.
Conclusions
Data from our study clearly indicate that short-term use of CFB in combination with chondroitin sulfate and glucosamine was effective in reducing knee discomfort and improving the physical mobility of the joints. Future investigations conducted with a larger cohort of subjects and for a longer duration; may provide better understanding of the short and long term effects of supplementation. The present study could be repeated as a crossover; in order to observe any effects of the combination therapy in subjects.
Acknowledgements: We express our gratitude to John Hunter and Brad Evers (FutureCeuticals Inc.) for their comments and suggestions in the preparation of this article. We would like to thank Mahalakshmi Babu for her help in editing the manuscript.
Disclosure Statement: The present study was funded by Futureceuticals, Inc. (Momence IL, USA). All authors declare that they have no conflicts of interest. No competing financial interests exist.
References
1. Porcheret M, Jordan K, Jinks C, Society PCicwtPCR. Primary care treatment of knee pain—a survey in older adults. Rheumatology. 2007;46(11):1694-700.
2. Frese T, Peyton L, Mahlmeister J, Sandholzer H. Knee Pain as the Reason for Encounter in General Practice. ISRN Family Medicine. 2013;2013:6.
3. Crofford L. Use of NSAIDs in treating patients with arthritis. Arthritis Research & Therapy. 2013;15(Suppl 3):S2.
4. Östör A, Watson PA. Topical NSAIDS provide effective pain relief for patients with hand or knee osteoarthritis with similar efficacy, and fewer side effects, than oral NSAIDS. Evidence Based Medicine. 2012.
5. Wang Y, Prentice LF, Vitetta L, Wluka AE, Cicuttini FM. The effect of nutritional supplements on osteoarthritis. Altern Med Rev. 2004;9(3):275-96. Epub 2004/09/25.
6. Ameye LG, Chee WS. Osteoarthritis and nutrition. From nutraceuticals to functional foods: a systematic review of the scientific evidence. Arthritis Res Ther. 2006;8(4):R127. Epub 2006/07/25.
7. Vandeweerd JM, Coisnon C, Clegg P, Cambier C, Pierson A, Hontoir F, et al. Systematic review of efficacy of nutraceuticals to alleviate clinical signs of osteoarthritis. Journal of Veterinary Internal Medicine. 2012;26(3):448-56.
8. Akhtar N, Haqqi TM. Current nutraceuticals in the management of osteoarthritis: a review. Therapeutic advances in musculoskeletal disease. 2012;4(3):181-207.
9. Lopez HL. Nutritional interventions to prevent and treat osteoarthritis. Part II: focus on micronutrients and supportive nutraceuticals. PM&R. 2012;4(5):S155-S68.
10. Ragle RL, Sawitzke AD. Nutraceuticals in the Management of Osteoarthritis. Drugs & aging. 2012;29(9):717-31.
11. Davidson EA, Meyer K. Chondroitin, a new mucopolysaccharide. The Journal of biological chemistry. 1954;211(2):605-11. Epub 1954/12/01.
12. Bana G, Jamard B, Verrouil E, Mazieres B. Chondroitin sulfate in the management of hip and knee osteoarthritis: an overview. Adv Pharmacol. 2006;53:507-22. Epub 2007/01/24.
13. Bruyere O, Reginster JY. Glucosamine and chondroitin sulfate as therapeutic agents for knee and hip osteoarthritis. Drugs & aging. 2007;24(7):573-80. Epub 2007/07/31.
14. Bucsi L, Poor G. Efficacy and tolerability of oral chondroitin sulfate as a symptomatic slow-acting drug for osteoarthritis (SYSADOA) in the treatment of knee osteoarthritis. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 1998;6 Suppl A:31-6. Epub 1998/09/23.
15. Clegg DO, Reda DJ, Harris CL, Klein MA, O’Dell JR, Hooper MM, et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. The New England journal of medicine. 2006;354(8):795- 808. Epub 2006/02/24.
16. Kubo M, Ando K, Mimura T, Matsusue Y, Mori K. Chondroitin sulfate for the treatment of hip and knee osteoarthritis: current status and future trends. Life sciences. 2009;85(13-14):477-83. Epub 2009/08/22.
17. Hart L. Are glucosamine and/or chondroitin sulfate effective for knee osteoarthritis? Clinical journal of sport medicine : official journal of the Canadian Academy of Sport Medicine. 2006;16(6):528-9. Epub 2006/11/23.
18. Uebelhart D, Malaise M, Marcolongo R, de Vathaire F, Piperno M, Mailleux E, et al. Intermittent treatment of knee osteoarthritis with oral chondroitin sulfate: a one-year, randomized, double-blind, multicenter study versus placebo. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2004;12(4):269-76. Epub 2004/03/17.
19. Mazieres B, Combe B, Phan Van A, Tondut J, Grynfeltt M. Chondroitin sulfate in osteoarthritis of the knee: a prospective, double blind, placebo controlled multicenter clinical study. The Journal of rheumatology. 2001;28(1):173-81. Epub 2001/02/24.
20. Ragle RL, Sawitzke AD. Nutraceuticals in the management of osteoarthritis : a critical review. Drugs & aging. 2012;29(9):717-31. Epub 2012/09/29.
21. Sawitzke AD, Shi H, Finco MF, Dunlop DD, Harris CL, Singer NG, et al. Clinical efficacy and safety of glucosamine, chondroitin sulphate, their combination, celecoxib or placebo taken to treat osteoarthritis of the knee: 2-year results from GAIT. Annals of the rheumatic diseases. 2010;69(8):1459- 64. Epub 2010/06/08.
22. Sawitzke AD, Shi H, Finco MF, Dunlop DD, Bingham CO, 3rd, Harris CL, et al. The effect of glucosamine and/or chondroitin sulfate on the progression of knee osteoarthritis: a report from the glucosamine/chondroitin arthritis intervention trial. Arthritis and rheumatism. 2008;58(10):3183-91. Epub 2008/09/30.
23. Attia M, Scott A, Carpentier G, Lian O, Van Kuppevelt T, Gossard C, et al. Greater glycosaminoglycan content in human patellar tendon biopsies is associated with more pain and a lower VISA score. British journal of sports medicine. 2013. Epub 2013/10/09.
24. Ishimaru D, Sugiura N, Akiyama H, Watanabe H, Matsumoto K. Alterations in the chondroitin sulfate chain in human osteoarthritic cartilage of the knee. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2013. Epub 2013/11/28.
25. Fransen M, Agaliotis M, Nairn L, Votrubec M, Bridgett L, Su S, et al. Glucosamine and chondroitin for knee osteoarthritis: a double-blind randomised placebo-controlled clinical trial evaluating single and combination regimens. Annals of the rheumatic diseases. 2014. Epub 2014/01/08.
26. Henrotin Y, Lambert C. Chondroitin and glucosamine in the management of osteoarthritis: an update. Current rheumatology reports. 2013;15(10):361. Epub 2013/08/21.
27. Aghazadeh-Habashi A, Jamali F. The glucosamine controversy; a pharmacokinetic issue. Journal of pharmacy & pharmaceutical sciences: a publication of the Canadian Society for Pharmaceutical Sciences, Societe canadienne des sciences pharmaceutiques. 2011;14(2):264-73. Epub 2011/07/08.
28. Bertozzi CR, Freeze HH, Varki A, Esko JD. Glycans in Biotechnology and the Pharmaceutical Industry. In: AC Varki, Richard D.; Esko, Jeffrey D., editor. Essentials of Glycobiology. 2nd edition ed. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2009.
29. Homandberg GA, Guo D, Ray LM, Ding L. Mixtures of glucosamine and chondroitin sulfate reverse fibronectin fragment mediated damage to cartilage more effectively than either agent alone. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2006;14(8):793-806.
30. Sherman AL, Ojeda-Correal G, Mena J. Use of glucosamine and chondroitin in persons with osteoarthritis. PM & R : the journal of injury, function, and rehabilitation. 2012;4(5 Suppl):S110-6. Epub 2012/06/01.
31. Miljkovic D, Scorei RI, Cimpoiasu VM, Scorei ID. Calcium fructoborate: plant-based dietary boron for human nutrition. Journal of dietary supplements. 2009;6(3):211-26. Epub 2009/01/01.
32. Reyes-Izquierdo T, Nemzer B, Gonzalez AE, Zhou Q, Argumedo R, Shu C, et al. Short-term Intake of Calcium Fructoborate Improves WOMAC and McGill Scores and Beneficially Modulates Biomarkers Associated with Knee Osteoarthritis: A Pilot Clinical Double-blinded Placebo-controlled Study. American Journal of Biomedical Sciences. 2012;4(2): p111-22.
33. Pietrzkowski Z, Phelan MJ, Keller R, Shu C, Argumedo R, Reyes-Izquierdo
T. Short-term efficacy of calcium fructoborate (CFB) on subjects with knee discomfort. A comparative, double blind, placebo-controlled clinical study. Clinical Interventions in Aging. 2014;9:895-9.
34. Scorei R. Is boron a prebiotic element? A mini-review of the essentiality of boron for the appearance of life on earth. Origins of life and evolution of the biosphere : the journal of the International Society for the Study of the Origin of Life. 2012;42(1):3-17. Epub 2012/04/25.
35. Scorei ID, Scorei RI. Calcium fructoborate helps control inflammation associated with diminished bone health. Biological trace element research. 2013;155(3):315-21. Epub 2013/08/29.
36. Militaru C, Donoiu I, Craciun A, Scorei ID, Bulearca AM, Scorei RI. Oral resveratrol and calcium fructoborate supplementation in subjects with stable angina pectoris: effects on lipid profiles, inflammation markers, and quality of life. Nutrition. 2013;29(1):178-83. Epub 2012/11/17.
37. Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. The Journal of rheumatology. 1988;15(12):1833-40. Epub 1988/12/01.
38. Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain. 1975;1(3):277-99. Epub 1975/09/01.
39. Melzack R. The McGill pain questionnaire: from description to measurement. Anesthesiology. 2005;103(1):199-202. Epub 2005/06/29.
40. Diggle PJH, P.; Liang, K.Y.; Zeger, S.L. . Analysis of Longitudinal Data. 2nd Edition ed. New York, NY, USA.: Oxford University Press; 2009.
41. Chung L, Chakravarty EF, Kearns P, Wang C, Bush TM. Bleeding complications in patients on celecoxib and warfarin. Journal of clinical pharmacy and therapeutics. 2005;30(5):471-7. Epub 2005/09/17.
42. Bush TM, Shlotzhauer TL, Imai K. Nonsteroidal anti-inflammatory drugs. Proposed guidelines for monitoring toxicity. The Western journal of medicine. 1991;155(1):39-42. Epub 1991/07/01.
43. Felson DT. Glucosamine and chondroitin sulfate in knee osteoarthritis: where now? Nature clinical practice Rheumatology. 2006;2(7):356-7. Epub 2006/08/26.
44. Baime MJ. Glucosamine and chondroitin sulfate did not improve pain in osteoarthritis of the knee. ACP journal club. 2006;145(1):17. Epub 2006/07/04.
45. Vassiliou VS. Glucosamine and chondroitin sulfate for knee osteoarthritis. The New England journal of medicine. 2006;354(20):2184-5; author reply -5. Epub 2006/05/20.
46. Pelletier JP. Glucosamine and chondroitin sulfate for knee osteoarthritis. The New England journal of medicine. 2006;354(20):2184-5; author reply -5. Epub 2006/05/20.
47. Ernst E. Glucosamine and chondroitin sulfate for knee osteoarthritis. The New England journal of medicine. 2006;354(20):2184-5; author reply -5. Epub 2006/05/19.
48. Wagner CC, Ferraresi Curotto V, Pis Diez R, Baran EJ. Experimental and theoretical studies of calcium fructoborate. Biological trace element research. 2008;122(1):64-72. Epub 2008/01/30.
49. Scorei RIC, C.; Ion, R.; Cimpean, A.; Galateanu, B.; Mitran, V.; Iordachescu, D. In vitro effects of calcium fructoborate upon production of inflammatory mediators by LPS-stimulated RAW 264.7 macrophages. Biological trace element research. 2010;135(1-3):334-44. Epub 2009/08/12.
50. Scorei R, Mitrut P, Petrisor I, Scorei I. A double-blind, placebo-controlled pilot study to evaluate the effect of calcium fructoborate on systemic inflammation and dyslipidemia markers for middle-aged people with primary osteoarthritis. Biological trace element research. 2011;144(1-3):253-63. Epub 2011/05/25.
51. Scorei IR. Calcium fructoborate: plant-based dietary boron as potential medicine for cancer therapy. Front Biosci (Schol Ed). 2011;3:205-15. Epub 2011/01/05.