M. Nunes-Pinto1,2, R.G. Bandeira de Mello2,3
1. Gerontopôle de Toulouse, Institut du Vieillissement, Centre Hospitalo-Universitaire de Toulouse, France; 2. Postgraduate Program in Medical Sciences (Endocrinology), Federal University of Rio Grande do Sul (UFRGS), Porto Alegre, Brazil; 3. Master of Public Health Program, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, USA
Corresponding Author: Mariá Nunes-Pinto, Gerontopôle de Toulouse, Institut du Vieillissement, Centre Hospitalo-Universitaire de Toulouse, France, nunespintomaria@gmail.com, +33744749342
J Aging Res & Lifestyle 2024;13:65-72
Published online May 22, 2024, http://dx.doi.org/10.14283/jarlife.2024.9
Abstract
Sarcopenia, a complex muscular condition driven by multi-systemic dysregulation and its interactions with lifestyle, physical attributes, and mental health, lacks effective drug treatments, relying primarily on non-pharmacological interventions. Fragmented approaches may prove suboptimal due to its complexity, underscoring the potential for multidomain interventions—a combination of two or more strategies to improve individual health—as a promising treatment option. This review examines the possible roles of multidomain interventions in sarcopenia, specifically addressing their effects on muscle mass and quality, muscle strength, and physical performance in older adults. While the updated literature highlights the beneficial consequences of multidomain interventions in enhancing physical performance outcomes, gaps persist in understanding their influence on the biological aspects of sarcopenia. Promising initial findings suggest changes in plasma inflammatory markers or muscle turnover networks, but further research is necessary to clarify the disease-modifying effects of multidomain intervention in sarcopenic patients.
Key words: Sarcopenia, multidomain interventions, non-pharmacological treatments, muscle health, disease-modifying effects.
Introduction
The Multidomain intervention is a broad term encompassing strategies involving at least two interventions, typically associating physical exercise, nutrition, cognitive training, or psychosocial components (1). Due to its simultaneous multiple targets, multidomain interventions are believed to promote comprehensive improvement in an individual’s health and prevent disability (2). Sarcopenia is a complex geriatric syndrome characterized by the progressive loss of muscle mass or quality, reduced strength, and declining physical performance (3). It is an age-related disease with systemic impacts, contributing to disability and mortality (4), thus making it a potential candidate for multidomain interventions. Although multidomain interventions may apply as a therapeutic option for sarcopenia, the extent to which they intervene on the pathophysiology of the disease (i.e., disease-modifying effect), on sarcopenia symptoms, or on both is unknown. The aim of this short review is to gather current data on the potential roles of non-pharmacological multidomain interventions in the treatment of sarcopenia.
Methods and Results
A comprehensive and sensitive search strategy was conducted in the PubMed database, combining multidomain and sarcopenia terms to identify relevant evidence in this field. We looked for randomized controlled trials (RCT) that operationalized a multidomain intervention in older people. Very limited research was uncovered regarding sarcopenic populations. Considering established connections between sarcopenia and frailty (5), we included studies enrolling participants with sarcopenia, pre-frailty or frailty, and mobility impairment (low short physical performance battery (SPPB) scores).
All RCTs evaluated older adults aged 65 years or older. The interventions, lasting from 12 weeks to 3 years, always comprised physical exercise, usually along with nutritional counseling or supplementation. Only two studies did not involve any nutritional strategy (6, 7). Additional components like cognitive training, psychosocial support, or comorbidities management were sometimes aggregated. Further details about included studies are summarized in Table 1.
Legend: IG Intervention group; CG Control group; n Number of participants; F female participants; mo Month; wk Week; x/ times per; HR Hazard ratio; ¹ 95% confidence interval; BGD Between-group difference; HGS Handgrip strength (kilogram); GS gait speed (meter per seconds); SPPB Short physical performance battery scores (points); 5CST Five chair stand test (seconds); TUG Timed Up and Go test (seconds); 400mt 400m walk test; KES Knee extension test (kilograms); ALM Appendicular lean mass (kilograms); ASMI Appendicular skeletal muscle mass index (ASM/height². Kilograms per meters²); CHS Cardiovascular Health Study; FNIH Foundation for the National Institutes of Health; PUFA Polyunsaturated fatty acid; IADL Instrumental Activities of Daily Living; IU International units; TNFα Tumor necrosis factor alpha (pg/mL); CSA Cross sectional area (cm²), RA radiological attenuation (Hounsfield units). Please refer to the original articles for further information.
The collected data refers to the effects of multidomain interventions in each of the components of sarcopenia (muscle mass/quality, muscle strength, or physical performance). For organizational clarity, the findings are here categorized into these three components in alignment with the consensus of the European Working Group on Sarcopenia in Older People (3). Finally, information related to the biological influences of multidomain interventions (disease-modifying effects) will be presented.
Physical performance
Regarding multidomain interventions in participants diagnosed with sarcopenia, two studies identified greater improvement in the SPPB total score in the multidomain intervention group compared to control group (8, 9). While one study found no differences between groups in gait speed (GS) after 12 weeks of intervention (10), Lu et al. identified that among the components of sarcopenia, GS exhibited the greatest change associated with multidomain intervention, with 22 out of 30 participants becoming free of low GS after 6 months (11). Moreover, in a multicenter European study involving 1519 older adults, a subgroup analysis of participants with lower SPPB score (3 to 7) revealed that the multidomain intervention was associated with a reduced risk of mobility disability (inability to perform the 400-meter walk test) during the 36-month follow-up (8).
Similar findings were seen among pre-frail/frail or mobility-impaired samples. Several studies identified greater improvement in the SPPB total score (6, 7, 12–15), GS (6, 12, 14–17) and timed-up-and-go test (TUG) (16, 17) in the multidomain intervention group compared to control groups. Only three studies found no difference between groups in GS (18, 19) and TUG (20).
Muscle strength
Examining samples with diagnosed sarcopenia, three studies evaluated muscle strength. Two of them found improved completion time for the 5-times chair sit-to-stand test (5CST) after the intervention compared to the control group (9, 10). Additionally, knee extension strength (KES) was greater in the intervention group than in the control group (10). While one study found no difference between groups in handgrip strength (HGS) after a two-year intervention (9), Bernabei et al. identified a smaller decline in HGS over the same period in women assigned to the multidomain intervention than those in the control group; however, no difference was seen among men (8).
In the two other populations, several studies also identified improvements in the 5CST (6, 12, 16, 20, 21) and lower limb strength (19, 20, 22) with the intervention compared to the control group, except for one study that did not find differences in KES between the groups (17). Casals et al. found a decrease in the prevalence of individuals with low HGS in the intervention group, with no significant changes observed in the control group (12). However, three other studies found no significant difference in HGS between groups (7, 17, 18).
Muscle mass and quality
Regarding individuals with sarcopenia, Zhu et al. identified increased appendicular skeletal muscle mass (ASM) index (ASM/height²) in the combined exercise-program-and-nutrition-supplement group over 12 weeks, while it decreased among controls (10). Bernabei et al. also found that women that received the multidomain intervention experienced less loss of appendicular lean mass (ALM) compared to the control group at 36 months. However, no significant differences were observed in men (8). Interestingly, in a sample of 92 sarcopenic individuals, among the three components of sarcopenia, muscle mass exhibited the least change, with only 7.6% achieving a normal ALM index (ALM/height²) after 6 months (11). In the other populations, one study observed a smaller decline in ASM index among the intervention group compared to controls. (6). Two other studies found no group differences concerning ALM (17, 22), although in one of them, the control group also received physical exercise (22).
More recently, studies have analyzed muscle quality parameters using image assessment techniques such as computed tomography scans (CT) or ultrasound (US). Performing US measurements on the vastus lateralis muscle of sarcopenic individuals, Monti et al. demonstrated preserved muscle architecture (pennation angle and fascicle length) despite a reduction in cross-sectional area (CSA) after the intervention. Conversely, in the control group, these architectural parameters declined along with a further decrease in CSA (9). Two studies assessed thigh measurements in mobility-impaired individuals. Within the intervention group, Englund et al. verified CT improvements in thigh composition (higher thigh muscle CSA, lower subcutaneous adipose tissue, lower intermuscular fat) (22). Skoglund et al. also observed significant increases in CT CSA and enhanced radiological attenuation, a parameter of muscle density, in knee extensors and hip adductors following the intervention. However, despite improvements in physical performance within this sample, multivariate analysis revealed that these changes were not directly associated with the alterations in muscle CSA and density post-intervention (15).
Biological evaluations
Ongoing research into the pathophysiology of sarcopenia reveals a complex multisystemic dysregulation indicative of altered muscle anabolic and catabolic responses, chronic inflammation, mitochondrial dysfunction, and neuromuscular changes (23). Two of the included studies assessed biological parameters after multidomain interventions in sarcopenic individuals. Through the analysis of blood T cells, Ma et al. discovered significant differences in the expression of seven genes associated with T cell regulation and inflammation (RASGRP1, BIN1, LEF1, ANXA6, IL-7R, LRRN3, and PRKCQ) between pre- and post-intervention measurements. These differences were found to correlate with leg extension strength (24). Moreover, Monti et al. found that C-terminal agrin fragment (CAF) levels, a marker of neuro-muscular junction (NMJ) instability, remained unchanged in the intervention group. At the same time, they increased in the control group after a two-year follow-up. They also observed a correlation between better SPPB scores and lower CAF concentration. Conversely, no difference was found in plasma levels of neurofilament light chain, another marker of the motoneuron health (9).
Other authors assessed biomarkers following multidomain interventions in pre-frail/frail populations. Concerning inflammation, Caldo-Silva et al. verified that plasma tumor necrosis factor alpha (TNF-α) levels decreased in the group receiving physical exercise and branched-chain amino acids supplementation, but only after a detraining period followed by a second wave of 16 weeks of intervention (21). Tan et al. also identified a reduction of TNF-α levels after 12 weeks of intervention compared to controls (6). No differences were seen among groups for other inflammatory indicators such as interleukin (IL) 6 (6), IL-10 (6, 21), and myeloperoxidase (21), nor in the hematological profile (hemoglobin, erythrocyte, white blood cell, or platelet counts) (25). Markers related to nutrient sensing and muscle turnover were also assessed. One study verified lower insulin-like growth factor (IGF) Binding Protein-3 to IGF-1 plasma ratios in the multidomain group compared to placebo, alongside reduced myostatin and increased brain-derived neurotrophic factor serum levels within the group (17). After the intervention, no difference was seen concerning growth differentiation factor 15 (6), Beta-2 microglobulin (17), albumin (21), and growth hormone (17).
Final considerations and future perspectives
Sarcopenia is a multifaceted geriatric syndrome with significant implications for both individuals and society, as it is associated with quality of life, independence, morbidity, and mortality (3). As of today, there is currently no evidence supporting drug treatments for sarcopenia; strength exercise training and nutritional support remain the main management options (26). Therefore, multidomain interventions are an interesting approach, especially when combining these last two aspects.
Findings from this review, summarized in figure 1, indicate beneficial impacts of multidomain interventions on the muscular components of sarcopenia among older adults. Muscle performance appears to be the more consistently improved aspect. Greater lower limb strength measured by CST or KES was also observed. Muscle mass seems to ameliorate with multidomain intervention, probably in a smaller degree compared to the other components. Conversely, the variability in improvements in HGS suggests a less uniform response. This could arise from several factors, including larger muscle mass volume, better response to interventions (27), or more pronounced age-related declines in lower limb muscle compared to the upper body (28). Moreover, lower limbs are extensively involved in weight-bearing and functional activities, and balance and mobility exercises often prioritize the lower body (29). Overall, responses seemed to be influenced by age, sex, degree of baseline impairment, and the type of intervention. Although not within this review’s scope, it’s important to recognize that since all studies combined physical exercise with another modality, and typically compared the intervention to a control group, the exclusive effect of physical exercise cannot be ruled out.
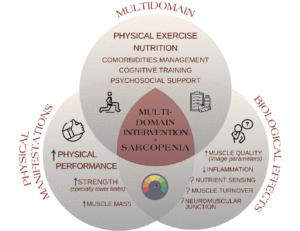
Figure 1. A summarized framework of multidomain interventions on sarcopenia and its components, including modifications in physical manifestations and potential biological effects, according to the main findings of our review
While multidomain interventions demonstrate a more consistent impact on the physical manifestations of sarcopenia, there is still limited understanding of their pathophysiology effects. Recent research delves into qualitative changes in the muscle, with modifications in architectural parameters and intramuscular fat being seen even without major changes in muscle mass. Furthermore, there may be primary effects concerning some inflammatory markers, nutrient sensing, and muscle turnover networks, or measurements of the neuromuscular junction. Still, current data is derived from a limited number of studies with small sample sizes, often lacking comprehensive analysis of biological parameters and their correlation with physical outcomes. Future research addressing larger populations aimed specifically at sarcopenic patients is needed, with a special focus on the biological primary pathways and disease-modifying effects of multidomain interventions.
Conflict of Interest: All authors declare no conflict of interest.
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
1. Bevilacqua R, Soraci L, Stara V, et al. A systematic review of multidomain and lifestyle interventions to support the intrinsic capacity of the older population. Front Med (Lausanne). 2022;9. doi:10.3389/FMED.2022.929261
2. Chen LK, Hwang AC, Lee WJ, et al. Efficacy of multidomain interventions to improve physical frailty, depression and cognition: data from cluster-randomized controlled trials. J Cachexia Sarcopenia Muscle. 2020;11(3):650-662. doi:10.1002/JCSM.12534
3. Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16. doi:10.1093/AGEING/AFY169
4. Chen LK, Woo J, Assantachai P, et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J Am Med Dir Assoc. 2020;21(3):300-307.e2. doi:10.1016/J.JAMDA.2019.12.012
5. Landi F, Cherubini A, Cesari M, et al. Sarcopenia and frailty: From theoretical approach into clinical practice. Eur Geriatr Med. 2016;7(3):197-200. doi:10.1016/j.eurger.2015.12.015
6. Tan LF, Chan YH, Seetharaman S, et al. Impact of Exercise and Cognitive Stimulation Therapy on Physical Function, Cognition and Muscle Mass in Pre-Frail Older Adults in the Primary Care Setting: A Cluster Randomized Controlled Trial. Journal of Nutrition, Health and Aging. 2023;27(6):438-447. doi:10.1007/S12603-023-1928-7
7. Stathi A, Greaves CJ, Thompson JL, et al. Effect of a physical activity and behaviour maintenance programme on functional mobility decline in older adults: the REACT (Retirement in Action) randomised controlled trial. Lancet Public Health. 2022;7(4):e316-e326. doi:10.1016/S2468-2667(22)00004-4
8. Bernabei R, Landi F, Calvani R, et al. Multicomponent intervention to prevent mobility disability in frail older adults: randomised controlled trial (SPRINTT project). BMJ. 2022;377. doi:10.1136/BMJ-2021-068788
9. Monti E, Tagliaferri S, Zampieri S, et al. Effects of a 2-year exercise training on neuromuscular system health in older individuals with low muscle function. J Cachexia Sarcopenia Muscle. 2023;14(2):794-804. doi:10.1002/JCSM.13173
10. Zhu LY, Chan R, Kwok T, Cheng KCC, Ha A, Woo J. Effects of exercise and nutrition supplementation in community-dwelling older Chinese people with sarcopenia: A randomized controlled trial. Age Ageing. 2019;48(2):220-228. doi:10.1093/ageing/afy179
11. Lu Y, Niti M, Yap KB, et al. Assessment of Sarcopenia Among Community-Dwelling At-Risk Frail Adults Aged 65 Years and Older Who Received Multidomain Lifestyle Interventions: A Secondary Analysis of a Randomized Clinical Trial. JAMA Netw Open. 2019;2(10). doi:10.1001/JAMANETWORKOPEN.2019.13346
12. Casals C, Ávila-Cabeza-de-Vaca L, González-Mariscal A, et al. Effects of an educational intervention on frailty status, physical function, physical activity, sleep patterns, and nutritional status of older adults with frailty or pre-frailty: the FRAGSALUD study. Front Public Health. 2023;11. doi:10.3389/FPUBH.2023.1267666
13. Cameron ID, Fairhall N, Langron C, et al. A multifactorial interdisciplinary intervention reduces frailty in older people: Randomized trial. BMC Med. 2013;11(1):1-10. doi:10.1186/1741-7015-11-65/TABLES/5
14. Fairhall N, Sherrington C, Lord SR, et al. Effect of a multifactorial, interdisciplinary intervention on risk factors for falls and fall rate in frail older people: a randomised controlled trial. Age Ageing. 2014;43(5):616-622. doi:10.1093/AGEING/AFT204
15. Skoglund E, Lundberg TR, Rullman E, et al. Functional improvements to 6 months of physical activity are not related to changes in size or density of multiple lower-extremity muscles in mobility-limited older individuals. Exp Gerontol. 2022;157. doi:10.1016/J.EXGER.2021.111631
16. Gené Huguet L, Navarro González M, Kostov B, et al. Pre Frail 80: Multifactorial Intervention to Prevent Progression of Pre-Frailty to Frailty in the Elderly. J Nutr Health Aging. 2018;22(10):1266-1274. doi:10.1007/S12603-018-1089-2
17. Kim H, Suzuki T, Kim M, et al. Effects of exercise and milk fat globule membrane (MFGM) supplementation on body composition, physical function, and hematological parameters in community-dwelling frail Japanese women: a randomized double blind, placebo-controlled, follow-up trial. PLoS One. 2015;10(2). doi:10.1371/JOURNAL.PONE.0116256
18. Kwon J, Yoshida Y, Yoshida H, Kim H, Suzuki T, Lee Y. Effects of a combined physical training and nutrition intervention on physical performance and health-related quality of life in prefrail older women living in the community: a randomized controlled trial. J Am Med Dir Assoc. 2015;16(3):263.e1-263.e8. doi:10.1016/J.JAMDA.2014.12.005
19. Ng TP, Feng L, Nyunt MSZ, et al. Nutritional, Physical, Cognitive, and Combination Interventions and Frailty Reversal among Older Adults: A Randomized Controlled Trial. American Journal of Medicine. 2015;128(11):1225-1236.e1. doi:10.1016/j.amjmed.2015.06.017
20. Rydwik E, Lammes E, Frändin K, Akner G. Effects of a physical and nutritional intervention program for frail elderly people over age 75. A randomized controlled pilot treatment trial. Aging Clin Exp Res. 2008;20(2):159-170. doi:10.1007/BF03324763
21. Caldo-Silva A, Furtado GE, Chupel MU, et al. Effect of training-detraining phases of multicomponent exercises and BCAA supplementation on inflammatory markers and albumin levels in frail older persons. Nutrients. 2021;13(4). doi:10.3390/NU13041106
22. Englund DA, Kirn DR, Koochek A, et al. Nutritional supplementation with physical activity improves muscle composition in mobility-limited older adults, the VIVE2 study: A randomized, double-blind, placebo-controlled trial. Journals of Gerontology – Series A Biological Sciences and Medical Sciences. 2018;73(1):95-101. doi:10.1093/gerona/glx141
23. Wiedmer P, Jung T, Castro JP, et al. Sarcopenia – Molecular mechanisms and open questions. Ageing Res Rev. 2021;65:101200. doi:10.1016/J.ARR.2020.101200
24. Ma SL, Wu J, Zhu L, et al. Peripheral blood T cell gene expression responses to exercise and HMB in sarcopenia. Nutrients. 2021;13(7). doi:10.3390/NU13072313
25. Caldo-Silva A, Furtado GE, Chupel MU, et al. Empowering frail older adults: multicomponent elastic-band exercises and BCAA supplementation unleash physical health and preserve haematological biomarkers. Front Sports Act Living. 2023;5. doi:10.3389/FSPOR.2023.1171220
26. Rolland Y, Dray C, Vellas B, Barreto PDS. Current and investigational medications for the treatment of sarcopenia. Metabolism. 2023;149. doi:10.1016/J.METABOL.2023.155597
27. Jung R, Gehlert S, Geisler S, Isenmann E, Eyre J, Zinner C. Muscle strength gains per week are higher in the lower-body than the upper-body in resistance training experienced healthy young women-A systematic review with meta-analysis. PLoS One. 2023;18(4). doi:10.1371/JOURNAL.PONE.0284216
28. Bullo V, Roma E, Gobbo S, et al. Lower Limb Strength Profile in Elderly with Different Pathologies: Comparisons with Healthy Subjects. Geriatrics. 2020;5(4):1-15. doi:10.3390/GERIATRICS5040083
29. De Labra C, Guimaraes-Pinheiro C, Maseda A, Lorenzo T, Millán-Calenti JC. Effects of physical exercise interventions in frail older adults: A systematic review of randomized controlled trials Physical functioning, physical health and activity. BMC Geriatr. 2015;15(1):1-16. doi:10.1186/S12877-015-0155-4/TABLES/2
© The Authors 2024

