E. Beattie1,2, J. McCrow1,2, C. Dyce3, E. Fielding2, E. Isenring3,4
1. Institute of Health and Biomedical Innovation, Brisbane, Queensland, Australia; 2. School of Nursing, Queensland University of Technology, Queensland, Australia; 3. Centre for Dietetics Research, School of Human Movement Studies, University of Queensland, Brisbane, Queensland, 4072, Australia; 4. Department of Nutrition and Dietetics, Princess Alexandra Hospital, Woolloongabba, Queensland
Corresponding Author: Elizabeth Beattie, School of Nursing, 6th floor, N Block, Queensland University of Technology, Kelvin Grove QLD 4059 Australia, elizabeth.beattie@qut.edu.au, Tel: +61 7 3138 3847, Fax: +61 7 3138 5941
Abstract
Background: The majority of people with dementia live at home until quite late in the disease trajectory, supported by family caregivers who typically take increasing responsibility for providing nutrition. Caregiving is highly stressful and thus both dyad partners are at risk of nutritional issues. Objective: This study evaluated the nutritional status of both dyad members and the associations between these. Design: Descriptive, correlational. Setting: Community. Participants: 26 dyads of persons with dementia and caregivers. Measurements: The nutritional status of each dyad member was evaluated at home using a comprehensive battery of measures including the Mini-Nutritional Assessment, Corrected Arm Muscle Area and a 3-day food diary. Stage of dementia and functional eating capacity was measured for the person with dementia. Caregivers completed a brief burden scale. Result: Of those with dementia (n = 26), a large proportion had nutritional issues (one was malnourished and another 16 were at risk). Six of the caregivers were at risk of malnutrition. In addition, fifteen of the people with dementia did not meet their recommended daily energy requirements. A moderate and significant positive correlation between functional eating skills and nutritional status (MNA score) among participants with dementia was found (r = .523, n = 26, p = .006). Conclusion: These findings suggest that a dyadic perspective on nutritional status provides important insights into risk in this vulnerable group. Specifically, monitoring of the functional eating independence skills of the person with dementia is critical, along with assisting caregivers to be aware of their own eating patterns and intake.
Key words: Dementia, nutritional status, caregiver, community, malnutrition.
Introduction
Poor nutritional status in the elderly population is an important predictor of morbidity and mortality (1). For older people living with dementia nutritional status is particularly important, given strong evidence that weight loss is associated with dementia, particularly Alzheimer’s Disease (2). An estimated 35.6 million people worldwide live with dementia, a number expected to double by 2030 and more than triple by 2050 (3). Dementia is considered to be a leading cause of dependency and disability among older people internationally (3).
Weight loss in persons with Alzheimer’s Disease begins up to six years prior to the diagnosis of dementia and accelerates one to two years before the onset of cognitive symptoms (2). This unintentional weight loss contributes to an increased risk of frailty, immobility, illness and premature morbidity (4). For people with dementia in whom adequate nutrition is not maintained, weight loss and malnutrition may occur in excess of that resulting from the disease process alone (5, 6). Protein- energy malnutrition, common among frail elders, is defined as insufficient dietary intake leading to an inadequate nutritional status, weight loss, and muscle wasting (1).
To date nutrition research has predominantly focused on the nutritional status of the person with dementia with more known about those living in residential care than in the community. However, the majority of people with dementia continue to live at home until quite late in the disease trajectory, supported by family caregivers, with caregivers typically taking increasing responsibility over time for food choices for the person with dementia as well as themselves. Typically the level of support required increases as the disease progresses (2).
Recent theoretical work has generated a concept analysis and model of mealtime difficulties in people with dementia, from an examination of 48 literature sources, again primarily from residential care settings (7). Important antecedents and attributes of mealtime difficulties include social and cultural factors, eating abits, mealtime patterns, environment, cognitive status and dyad interaction. As the extent of assistance needed increases, the associated impact on the caregiver as well as the person with dementia is significantly increased.
Given that caring for a person with dementia is highly stressful, and is associated with physical and emotional burden (8) and poor nutrition (9, 10), it is important to understand more about the nutritional status of both the caregiver and the person with dementia within the dyadic relationship. Previous observational research has shown that caregivers eat fewer than two meals per day however little is known about the actual nutritional status of family caregivers (11).
Collectively these findings support the need for further research investigating dyadic nutritional status as potentially there may be some evolving dyadic nutritional profiles. This descriptive, cross-sectional study reports on the nutritional status of community-dwelling people with dementia and their family caregiver, addressing this gap.
Specifically, our research questions were:
-
What is the nutritional status and dietary intake (and associations between these) of community-dwelling dyads of caregivers and people with dementia?
-
What associations exist between level of cognitive impairment and nutritional status for the person with dementia?
It was hypothesized that both family caregivers and the person with dementia would be at risk of nutritional issues and there would be a dyadic nutritional relationship.
Methods
Participants
The participants were community-dwelling people with dementia and their adult family caregivers, located in South East Queensland, Australia. Potential participants were initially identified through a variety of mechanisms including: community home care workers, respite centre managers/coordinators and multimedia advertisements. Inclusion criteria were: care recipient and the caregiver relative living in the same household; person with dementia aged 55+ with a diagnosis of dementia and independently ambulating. To be eligible the caregiver must have reported that they prepared at least ten meals per week for their relative. People with dementia were excluded if they had a concurrent disease, such as cancer, causing unintentional weight loss.
Approval for this project was obtained from the Human Research Ethics Committees of all relevant universities, health districts and facilities. Written informed consent for participation was provided by the participants and/or the responsible relative or legal guardian.
Measures/ instruments
All participants completed an assessment battery including a demographic questionnaire.
For both dyad members
Nutritional status was assessed using the Mini Nutritional Assessment [MNA] (12).
Body weight and fat and fat-free mass were assessed using electronic Tanita scales.
Corrected Arm Muscle Area [CAMA], an indicator of nutritional status in terms of body protein and fat stores, was calculated from mid-upper arm circumference and tricep skin fold [TSF]. Triplicate TSF measurements (to the nearest 0.2mm) were made using a standard procedure (Harpenden calipers) (13).
A 3-day food diary was recorded by the caregiver on two week days and one weekend day (14). Due to the cognitive decline issues in this group, it was decided that dietary intake assessment methods relying on recall were not the best choice. In addition 3-day diet diary has been shown to be valid and acceptable for this older age group (15). Participants utilized a simple instruction booklet developed by the study dietician for recording food and fluid type with specified portions. Experienced RAs assisted the caregiver with checking and completing the diaries. When participants did not list all details, some common assumptions were used, such as: generic products were selected when no product brand was recorded; preparation of common meals were assumed when details were not specified (such as sandwiches, stews and curries); and portion sizes were based on previous meals when not recorded in detail.
For the person with dementia
Global cognitive status was quantified using the Mini- mental Status Exam [MMSE] (16). The MMSE yields a global performance score (maximum 30) from eleven, absolute anonymity & fast. items measuring orientation, registration, attention and calculation, recall, language and construction tasks.
Stage of dementia was assessed using the Clinical Dementia Rating Scale [CDR] (17), completed by the caregiver.
Functional self-eating skill of the person with dementia was assessed using the Eating Behavior Scale [EBS] over three meal times. This six item scale measures self-eating in five dimensions, with a total score of 18: attention to meal; ability to locate food; use of utensils; biting, chewing and swallowing, and ending the meal (18).
For the Caregiver only
Pearlin’s four-item scale was used to assess the strain (burden) of caring for someone with dementia (e.g. you are exhausted when you go to bed at night). Caregivers rated each item on a 4-point scale from 1 (not at all) to 4 (completely) (19).
Procedures
Following informed consent, dyads were mailed a package with several of the instruments to be filled out in their own time. Other instruments were collected face-to- face during a 90-minute home visit by two research assistants. At this visit, anthropometrics (height and weight) were taken and household measuring implements (cups, spoons, liter jug, electronic food scale) were left for the caregiver to complete the three-day food and fluid diary. A follow-up phone call was made with each caregiver to answer any emerging questions they may have had about completing the food and fluid diary.
Statistical Analyses
All analyses were performed using the Statistical Package for the Social Sciences (SPSS) version 21. Daily mean protein and energy content was calculated from the three-day record using Australian food composition data (Food Works Professional version 7, Xyris software, Brisbane). Percentages of estimated energy and protein requirements met [EER and EPR] for each participant were calculated using a conservative value of 114kJ/kg (26kcal/kg) and 0.9g/kg of body weight. Global CDR was derived from the scores following the original CDR guidelines (17).
Descriptive statistics were used to summarize characteristics of the sample and frequency calculations were used to summarize the categorical validation response variables. Pearson correlations were used to test the following associations: within person between nutritional status and energy and protein intake; for the person with dementia between nutritional status and cognitive status and functional eating status; and within- dyad between the nutritional status of caregiver and person with dementia. Statistical significance was at the standard P
Results
A total of 32 dyad participants responded to our recruitment requests. Of these, one was excluded because of the advanced state of dementia, two dyads declined to participate, two failed to return consent forms and one dyad withdrew because of a serious medical event. A sample of 26 dyads was included in the final analyses.
The spouse of the person with dementia (n = 21) was the most reported caregiver relationship, with other family members including grandchildren and children making up the rest of the sample. The median time of care-giving was 51 months (range 7-156 months). However, most caregivers (n = 21) had been formally caring for the person with dementia for three years or longer.
The type of dementia for half of the participants was Alzheimer’s (n = 13), with five having vascular, one having Lewy body and one early onset (the remaining six were unsure as to specific type). Results from the CDR indicated that participants were in the following stages of dementia: eleven mild, eight moderate, and three severe, with four having questionable dementia.
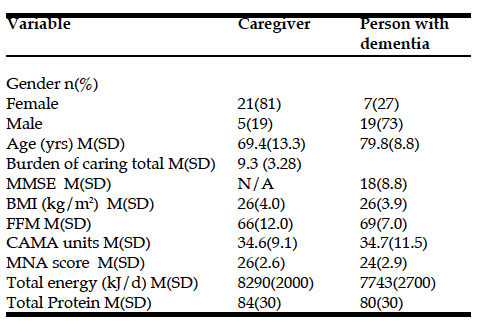
In terms of caregiver burden, the mean score was 9 out of a maximum of 16. The most frequently reported strain was that they were “exhausted when they went to bed at night”—half (13 out of 26) answered ‘quite a bit’ or ‘completely.’ In addition, eleven caregivers chose one of those two categories for “did not have time just for themselves.” Other demographic and clinical characteristics of the participants are presented in Table 1.
MMSE = The Mini Mental State Examination; BMI = Body Mass Index; FFM = Fatfree
Mass; CAMA = Corrected Arm Muscle Area; MNA = Mini-Nutritional Assessment
Question one: Nutritional status and dietary intake
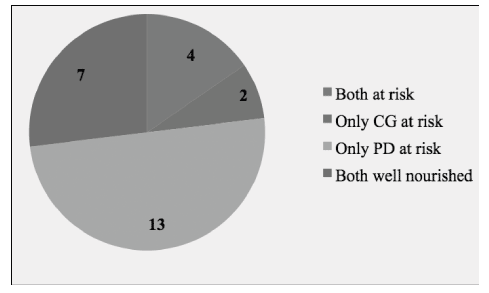
Results from the MNA indicated that one of the people with dementia was malnourished and 16 (out of 26) were at risk of malnutrition. None of the caregivers were identified as malnourished, while six were at risk of malnutrition. Figure 1 displays the distributions of the nutritional profiles of the participants as measured with the MNA. When assessing the dyad nutritional status, again with MNA, results indicated that four of the dyads were both at risk of malnutrition and seven were both well nourished. For the remaining, two dyads had only the caregiver at risk of malnutrition, while only the person with dementia was at risk in half (n = 13) of the sample (see Figure 2).
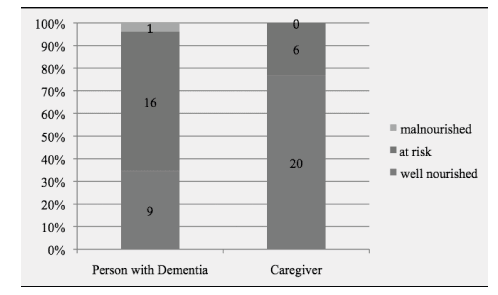
Figure 1: Nutritional Profiles of Participants. MNA indicator scores: less than 17 points = malnourished; 17 to 23.5 points = at risk of malnutrition; 24 to 30 points = normal nutritional status.
Of the total sample, one dyad could not complete the three day food diary due to comprehension difficulties. Of the caregiver participants, the mean reported daily energy and protein intake was 8300 kJ (± 2000) and 80g (±
30) respectively (Table 1). The dementia sample reported a mean daily energy intake of 7700 kJ (± 2700) and a mean protein intake of 80g (± 29). Of the caregiver sample, nine participants did not meet their EER and six participants did not meet their EPR. Fifteen (of 26) of the participants with dementia did not meet their EER and nine did not meet their EPR.
In assessing whether there was a relationship between the nutritional status of the caregiver and the person with dementia within a dyad, bivariate tests were computed for each variable. The only two nutritional variables showing a significant within-dyad association was for the percent of EER (and the percent of EPR) met by the diary food intake of the caregiver and the person with dementia (EER: r = .463, n = 25, p = .020; EPR: r = .514, n = 25, p = .009).
There was a moderate positive relationship between CAMA scores and protein intake in the caregiver sample (r = .41, n = 25, p = .043) and between CAMA and energy (r = .45, n = 25, p = .025) and CAMA and protein (r = .40, n = 25, p = .046) among the people with dementia. There were no statistically significant relationships between MNA scores and protein and energy intake for either group. Similarly, none of the relationships between meeting EER and EPR and CAMA and MNA status were statistically significant. However, among the participants with dementia, the correlation between functional eating skills and nutritional status (MNA score) was positive, moderate in size and significant (r = .523, n = 26, p =.006).
Question two: relationship between cognitiveimpairment and nutritional status
For the people with dementia Pearson’s r was computed to assess the relationship between MNA scores and level of cognition as rated by the MMSE. There was no significant correlation between the two variables (r =.26, n = 26, p = .206).
Discussion
Summary of key findings
This study provides a description of the nutritional status of community-dwelling dyads of people living with dementia and caregivers. In the community, 15% of older people are at risk of malnutrition with an additional 5-30% being malnourished (1, 20). The striking finding in this study was the very high (16 of 26) proportion at risk of malnutrition among the participants with dementia compared to normative rates in the non-caregiver non- dementia diagnosed population with a similar age profile (21). In addition, almost a quarter of family caregivers (n = 6) presented as at risk of malnutrition. These results are in line with the small number of existing studies of community-dwelling people with dementia. In the only other study (n = 56) to look at dyads, Rullier et al (2012) found a similar prevalence (59%) of people with dementia being at risk of malnutrition, but a higher prevalence (23%) being malnourished (22). In Rullier et al’s sample, more of the family caregivers displayed nutritional issues; nearly a third (32%) were at risk of malnutrition while 5% were malnourished. Two other studies examined only people with dementia; a large study (n = 940) resulted in quite similar malnutrition prevalence rates to the current study (5% malnourished and 43% at risk) (23) and a smaller study (n = 49) identified 43% of dementia participants as malnourished or at risk (24).
Our study found an alarmingly high prevalence of suboptimal protein and energy consumption, especially in people with dementia. Because our estimates of energy and protein requirements were conservative (0.9kg/kg) these results are likely to underreport the percentage of subjects meeting actual requirements (i.e. if 1.2g/kg had been used). These results largely support current literature, which infers that poor dietary intake is prevalent among older people with cognitive impairment (25). Energy and protein are vital nutrients and key to the maintenance of health in older adults.
An important association linking eating behavior disturbances and the risk of malnutrition is highlighted by this study. Our results indicate diminished independence of eating behaviors is strongly related to the risk of malnutrition. Those participants whose dementia most affected their eating abilities also tended to be at most risk of malnutrition. Many studies have investigated the role of nutrition on global functioning, showing an important association between malnutrition and functional decline (25). Cognitive disorders commonly influence the individual’s ability to function independently (26).
There are a number of risk factors that have been suggested to contribute to nutritional outcomes of people living with dementia, including severity of cognitive impairment and the timing of the formal dementia diagnosis (25). Conversely, Rullier et al (2012) found no association between malnutrition and cognitive severity (22), as in our results. Rullier et al hypothesized that it is primarily the role of specific activities of daily living affected by the trajectory of the disease and not solely diminished cognitive impairment that impacts nutritional status. Our sample comprised a wide range of cognitive impairment, and the diversity in individual symptoms and characteristics could also explain the results.
Implications for clinical practice
Our results suggest this vulnerable population would benefit from systematic dietary assessment and intervention to prevent further inadequacy in dietary intake and increased nutritional risk, consistent with recommendations by Shatenstein, Kergoat & Reid (2007) (25). Simple nutritional screening can help identify not only people with dementia at nutritional risk but their co- dwelling caregivers with similar risk. A comprehensive appraisal of the level of independence of the person with dementia in the context of eating behaviors is particularly important. Prioritizing eating behavior autonomy, and evaluating when and how to provide eating assistance, may prove to be the most effective intervention to maintain nutritional status of the caregiving dyad. Caregivers and dietetic professionals need to be aware that dyads are at particularly high risk of suboptimal protein and energy intake and that focusing only on the person with dementia or only on the caregiver may provide a limited view of the situation. Keller et al (2007) argues that the interactions among the caregiving dyad during the feeding-related activities, including mealtimes, are central to the nutritional status of both members of the dyad. This underlines the importance of not only assessing eating behavior autonomy, but highlights that for the most effective nutritional interventions a multi-faceted approach that prioritizes eating autonomy may offer the most productive outcome.
Limitations and directions for future research
The relatively small sample size is a primary limitation of this study. However since this is only the second published study of dyads found in the literature, it provides important insights into the challenges of dyad recruitment and measurement in nutrition-focused dementia studies. Future research needs to explore the role of specific symptoms associated with cognitive impairment and caregiver challenges that may contribute to increased risk of poor nutritional outcomes. Increased knowledge of how dyads operate at home at mealtimes and how food is decided upon, prepared and shared is also important in understanding the dynamics of malnutrition in these vulnerable pairs. This, in turn, may allow for the development of more effective interventions to delay or prevent malnutrition.
Not only are persons with dementia at high risk of malnutrition but also their co-dwelling caregivers. It is evident that this population is at risk of suboptimal energy and protein intake and therefore and that those caregivers need to be mindful of their own vulnerability to nutritional issues.
Acknowledgements: The authors would like to acknowledge the 26 caregiver- person with dementia dyads that freely gave their time to participate in this study. Recruitment would not have succeeded without the assistance of the many aged care providers involved. In addition, able research assistance was provided by Joan Connor, Daniel Lee, and Margaret MacAndrew.
Funding: This project was funded the Alzheimer’s Australia Hazel Hawke Research Grant in Dementia Care. The sponsors had no role in the design and conduct of the study; in the collection, analysis, and interpretation of data; in the preparation of the manuscript; or in the review or approval of the manuscript.
References
-
Watterson C, Fraser A, Banks M, et al. Evidence based practice guidelines for the nutritional management of malnutrition in adult patients across the continuum of care. Nutr Diet 2009; 66:S1-S34.
-
Johnson DK, Wilkins CH, Morris JC. Accelerated weight loss may precede diagnosis in Alzheimer disease. Arch Neurol 2006; 63:1312- 7.
-
World Health Organization. Dementia: A public health priority. 2012. Geneva: WHO.
- White H, Pieper C, Schmader K. The association of weight change in Alzheimer’s disease with severity of disease and mortality: a longitudinal analysis. J Am Geriatr Soc 1998; 46:1223- 7
-
Morley JE. Dementia is not necessarily a cause of undernutrition. J Am Geriatr Soc 1996; 44:1403-4.
-
Franzoni S, Frisoni GB, Boffelli S, Rozzini R, Trabucchi M. Good nutritional oral intake is associated with equal survival in demented and nondemented very old patients. J Am Geriatr Soc 1996; 44:1366-70.
-
Aselage MB, Amella EJ. An evolutionary analysis of mealtime difficulties in older adults with dementia. J Clin Nurs 2010; 19:33-41.
-
Vitaliano PP, Young HM, Zhang J. Is caregiving a risk factor for illness? Curr Dir Psychol Sci 2004; 13:13-6.
-
Mujuru NG. The psychosocial impact of care-giving on the family caregivers of chronically ill AIDS/HIV patients in home based care. 2010. Master’s Thesis: University of the Witwatersrand, Johannesburg.
-
Branscum A. Stress and coping model for family caregivers of older adults. 2010. Dissertation: University of Iowa.
-
Rabinowitz YG, Gallagher-Thompson D. Health and health behaviors among female caregivers of elderly relatives with dementia. Clin Gerontologist 2007; 31:1-15.
-
Guigoz Y, Vellas B, Garry P. Mini Nutritional Assessment: a practical assessment tool for grading the nutritional state of elderly patients. In: Vellas B, Guigoz Y, Garry P, Albarede J (eds) The mini nutritional assessment: MNA. Nutrition in the elderly. 1997. Paris, Serdi, pp 15-60.
-
Friedman PJ, Campbell JA, Caradoc-Davies TH. Prospective trial of a new diagnostic criterion for severe wasting malnutrition in the elderly. Age Ageing 1985; 14:149-54.
-
Gibson RS. Principles of nutritional assessment 2nd ed. 2005. Oxford USA, Oxford University Press.
-
Rothenberg E. Experience of dietary assessment and validation from three Swedish studies in the elderly. Eur J Clin Nutr 2009; 63:S64-8.
-
Folstein M, Folstein S, McHugh P. “Mini-Mental State”: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189-98.
-
Morris JC. Clinical dementia rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int Psychogeriatr 1997; 9:173-6.
-
Tully MW, Matrakas LK, Muir J, Musallam K. The Eating Behavior Scale. A simple method of assessing functional ability in patients with Alzheimer’s disease. J Gerontol Nurs 1997; 23:9-15.
-
Pearlin LI, Mullan JT, Semple SJ, Skaff MM. Caregiving and the stress process: An overview of concepts and their measures. Gerontologist 1990; 30:583-94.
-
Leggo M, Banks M, Isenring E, Stewart L, Tweeddale M. A quality improvement nutrition screening and intervention program available to Home and Community Care eligible clients. Nutr Diet 2008; 65:162-7.
-
Rist G, Miles G, Karimi L. The presence of malnutrition in community-‐living older adults receiving home nursing services. Nutr Diet 2012; 69:46-50.
-
Rullier L, Lagarde A, Bouisson J, Bergua V, Barberger-‐Gateau P. Nutritional status of community-‐dwelling older people with dementia: Associations with individual and family caregivers’ characteristics. Int J Geriatr Psychiatry 2013; 28: 580-8.
-
Roqué M, Salva A, Vellas B. Malnutrition in community-dwelling adults with dementia (Nutrialz trial). J Nutr Health Aging 2013; 17:295-9.
-
Spaccavento S, Del Prete M, Craca A, Fiore P. Influence of nutritional status on cognitive, functional and neuropsychiatric deficits in Alzheimer’s disease. Arch Geront Geriatr 2009; 48:356-60.
-
Shatenstein B, Kergoat M-J, Reid I. Poor nutrient intakes during 1-year follow-up with community-dwelling older adults with early-stage Alzheimer dementia compared to cognitively intact matched controls. J Am Diet Assoc 2007; 107:2091-9.
-
Del Parigi A, Panza F, Capurso C, Solfrizzi V. Nutritional factors, cognitive decline, and dementia. Brain Res Bull 2006; 15; 69:1-19.