T. Reyes-Izquierdo1, C. Shu1, R. Argumedo1, B. Nemzer2, Z. Pietrzkowski1
-
Applied BioClinical Inc., 16259 Laguna Canyon Rd, Irvine, CA, USA 92618;
-
FutureCeuticals Inc., 2692 N. State Rt. 1-17., Momence, IL, USA 60954
Corresponding Author: Tania Reyes-Izquierdo, 16259 Laguna Canyon Rd, Irvine CA, 92618 USA, Phone +1 949 502 4496, Fax +1 949 502 4987, Email: tania@abclinicaldiscovery.com
Abstract
Purpose: elevATP™, a supplement containing plant-derived inorganic microelements and apple polyphenols, was previously shown to increase endogenous whole blood ATP levels in healthy human subjects. In this report, we tested the supplement in a larger cohort and assessed the effect of the supplement in muscle. Methods: Twenty healthy, fasted, and resting adult human subjects participated in this acute, placebo-controlled, single-dose crossover clinical study. Oral placebo was administered on the first day of testing followed by a single, 150 mg dose of elevATP™ on the second day. Blood was collected immediately prior to treatment, 60 and 120 minutes after ingestion. Whole blood ATP, plasma ATP, hemoglobin, blood lactate, and blood glucose levels were collected. A muscle biopsy was performed on one resting study subject before, and 60 and 120 minutes after, a single dose of elevATP™. Results: elevATP™ increased whole blood levels of ATP by 40% after 60 minutes (p
Key words: Blood ATP, plasma ATP, micronutrients, polyphenols.
Introduction
Adenosine 5’-triphosphate (ATP) is the active source of energy within cells and participates in numerous physiological processes. Extracellular ATP and ADP influence platelet aggregation, vascular tone, and nervous, cardiac and muscle tissue function (1). ATP is critical for cell-to-cell communication and participates in immune cell coordination (2-5). As humans age, intracellular ATP levels decrease and the ability to generate ATP is diminished (6-8). Recent evidence also suggests that aging humans have lower plasma and erythrocyte-mediated release of ATP, most notably during periods of increased skeletal muscle blood flow (9). This may impair vasodilation and oxygen delivery to the tissues (9).
The clinical effects of oral ATP administration have been mixed (10, 11). Researchers have failed to demonstrate that orally administered ATP supplements
increase blood ATP levels (11, 12). This is likely due to the poor bioavailability of oral ATP (13), since intravenous administration of ATP has been reported to increase ATP levels (14). Long term ATP supplementation may induce intestinal nucleoside transporters in humans, thereby increasing absorption (15). However, enteric-coated and distal-releasing ATP supplements have also failed to increase blood ATP levels (12). Because of the unpredictable mechanism of ATP dosing using direct ATP supplementation, researchers, including our group, are exploring strategies to increase endogenous ATP levels.
ElevATP™ is an ancient peat-based bioinorganic material blended with apple extract polyphenols, as previously described (16). Our pilot study demonstrated that a single dose of elevATP™ increased whole blood ATP levels in human subjects without changing reactive oxygen species, glucose, or hytrin pills lactate levels in the blood (16). This study extends that work using a crossover design in a larger group of subjects. We sought to confirm the results of our pilot study and to evaluate plasma levels of ATP as well. In addition, biopsy of muscle tissue was collected from one individual before and after a single dose of elevATP™ in order to determine intramuscular ATP levels.
Materials and Methods
elevATP™ was provided by FutureCeuticals, Inc., Momence, IL USA. Dulbecco’s phosphate buffered saline (PBS), phenyl methane-sulfonyl-fluoride (PMSF), dimethyl sulfoxide (DMSO), leupeptin, EDTA, NaCl, nitrobenzyl thioinosine (NBTI), KCl, tricine, forskolin, isobutylmethylxanthine (IBMX) and water were purchased from Sigma Chem. Co. (St Louis, MO, USA). ATP stabilizing solution was prepared as described by Gorman et al. (17) (118 mmol NaCl, 5 mmol KCl, 40 mmol dec 26, 2014 – dec 6, 2013 – product name: lioresal . active ingredient: baclofen . category: muscle relaxants. thumbnail sketch: generic lioresal is used for tricine buffer, 4.15 mmol EDTA, 5 nmol NBTI, 10 µmol forskolin and 100 µmol IBMX, at pH 7.4 adjusted with 2 mol/L KOH).
Low protein binding microtubes were obtained from Eppendorf (Hauppauge, NY, USA) and RC DC Protein Assay Kit II was obtained from Bio-Rad (Palo Alto, CA, USA). ATP-luciferase assays were obtained from Calbiochem (San Diego, CA, USA). Heparin capillary blood collection Cymbalta tubes were obtained from Safe-T-Fill® (Ram Scientific Inc. Yonkers, NY). A portable gas meter and CG8+ cartridges were obtained from Abbott Laboratories (Abbott Park, IL, USA). Total hemoglobin quantification ELISA kits were obtained from MyBiosource (San Diego, CA, USA). Accutrend® Lactate Point of Care and BM-Lactate Strips® were obtained from Roche (Mannheim, Germany). Accu-Chek® Compact Plus glucometer and Accu-Chek® test strips were obtained from Roche Diagnostics (Indianapolis, IN, USA).
Clinical Study
Inclusion and Exclusion Criteria
This clinical case
study was conducted according to guidelines laid out in the Declaration of Helsinki. All procedures involving human subjects were approved by the Institutional Review Board at Vita Clinical S.A. Avenida Circunvalacion Norte #135, Guadalajara, JAL, Mexico 44 270 (study protocol no. ABC-13-09-ATP). Twenty subjects were selected to participate. They were generally healthy, and free of rhinitis, influenza, and other acute infections. 12 female and 8 male subjects were selected, with ages ranging from 22 to 35 years and BMI ranging from 24.1 to 30 kg/m². Exclusion criteria included diagnosis of diabetes mellitus, allergies to dietary products, use of anti-inflammatory drugs, analgesics, statins, diabetic drugs, anti-allergy medicines, multivitamins, and use of supplements within 15 days of the start of the study. All participants gave written, informed consent before any experimental procedure was performed.
Blood Collection
Enrolled participants were instructed not to eat for 12 h prior to the initial blood draw. Resting subjects were given an empty capsule as placebo on Day 1 of the study and 150 mg of encapsulated elevATP™ on Day 2. 250 mL of water was administered with the capsules each day. Two hundred microliters of blood was collected by finger puncture and placed in Safe-T-Fill® Capillary blood collection tubes (Ram Scientific Inc. Yonkers, NY). Blood samples were collected immediately prior to test capsule administration and at 60 and 120 minutes after ingestion.
Plasma Collection for ATP analysis
One hundred µl of blood was transferred to low- protein binding tubes (Eppendorf, Hauppauge, NY, USA) immediately after collection. An equal volume of ATP stabilizing solution (17) was added to each tube. Tubes were gently mixed by inversion and centrifuged at 13,000 g for 3 min to pellet cells. Supernatant was transferred to a clean tube and spun again at 13,000 g for 3 min. The supernatant was then snap frozen and stored at -80°C prior to ATP analysis.
ATP Detection and Quantification
Blood ATP or plasma ATP concentration were determined using ATP Assay Kits (Calbiochem, San Diego, CA, USA) with a modification to the original method, as previously described[18]. Briefly, 10 μL of lysed blood or plasma was loaded onto a white plate (Corning® Fisher Scientific, Waltham, MA, USA). 100 µL of ATP nucleotide-releasing buffer containing 1 µL luciferase enzyme mix was added and the plate immediately placed on a illuminometer (LMaX, Molecular Devices; Sunnyvale CA, USA). Readings were performed for 15 min at 3 min intervals, at 470 nm. Relative Light Units (RLU) were recorded and ATP concentrations were determined using a standard ATP curve.
Hemoglobin Measurement
Hemoglobin levels in plasma were determined using a double sandwich ELISA (MyBiosource, San Diego, CA, USA), concentration was determined comparing to a standard curve, according to the manufacturer’s instructions. Plasma samples collected with the ATP stabilizing solution were used for this analysis.
Lactate and Glucose Detection
Blood lactate was measured using an Accutrend® Lactate Point of Care (Roche, Mannheim, Germany) and BM-Lactate Strips® (Roche, Mannheim, Germany). Fifteen µL of blood was loaded onto the strip and lactate levels were read according to the manufacturer’s instructions. Glucose was measured using an Accu-Chek® Compact Plus glucometer (Roche Diagnostics, Indianapolis, IN, USA) and Accu-Chek® test strips (Roche Diagnostics, Indianapolis, IN, USA). Glucose was read according to the manufacturer’s instructions. Lactate and glucose levels were determined at every collection time point.
Statistical Analysis
For each result obtained from the described assays, each subject was normalized to their own value measured at baseline (T0), before ingestion of elevATP™ or placebo. Levels of each assay at 60 (T60) and 120 (T120) minutes after treatment were compared within experimental groups to the baseline and between experimental groups using a paired t-statistic test. Descriptive analyses were run in GraphPad® to derive the mean and standard deviation of each group.
Muscle Biopsy and Surgical Procedure
For the muscle biopsy, one twenty two-year old healthy subject, with a BMI of 24.5 was recruited, following the same selection criteria as described for the clinical crossover study. This clinical case study was conducted according to guidelines laid out in the Declaration of Helsinki. This procedure was approved by the Institutional Review Board at Vita Clinical S.A. Avenida Circunvalacion Norte #135, Guadalajara, JAL, Mexico 44 270 (study protocol no. ABC-NCI-13-01-ATP- Mus1). The study subject was selected from the group of subjects enrolled for whole blood ATP measurement, as described above. This subject was fasted and resting during this experiment. Biopsy was performed using aseptic technique. An antiseptic solution (Isodine) was applied to the medial region of the arm, over the right biceps. An 18 g needle was used to infiltrate the skin with 5 cc lidocaine. A skin incision was made using a 3 mm skin biopsy punch. Subcutaneous tissue was bluntly divided, allowing resection of 3 mm2 of biceps muscle using Metzenbaum scissors. Muscle tissue from the biceps was collected before, and also 60 and 120 minutes after, ingestion of elevATP™. Muscle tissue was deposited in a 50 ml conical tube and frozen using liquid nitrogen prior to further processing needed for measuring of ATP.
Measurements of ATP in muscle tissue
Frozen muscle tissue was added to a glass tissue grinder (Fisher Scientific, Chino, CA, USA) containing 200 μL ice cold ATP stabilizing solution, as previously described by Gorman et al. (17). Tissue was mechanically ground and transferred to a low-protein binding microtube (Eppendorf, Hauppauge, NY, USA). The sample was centrifuged for 5 min at 10,000 g and upernatant was used for ATP quantification. ATP concentration was determined using an ATP Assay Kit (Calbiochem, San Diego, CA, USA) with a modification to the original method, as previously described (18).
Hemoglobin levels were also determined in muscle tissue lysates, using a double sandwich ELISA (MyBiosource, San Diego, CA, USA), according to the manufacturer’s instructions. Tissue samples homogenized in ATP stabilizing solution described by Gorman et al. (17) were used for this analysis.
Results
Twenty healthy subjects were recruited for this placebo-controlled, crossover study. Subjects fasted overnight and were then given an empty capsule as placebo (Day 1). Blood was collected at baseline (before treatment) and 60 min (T60) and 120 min (T120) after treatment. Subjects fasted overnight, prior to Day 2, when a single capsule containing 150 mg of elevATP™ was administered to each subject. Blood was obtained as previously described. Blood ATP and glucose, and plasma ATP and hemoglobin levels were also determined.
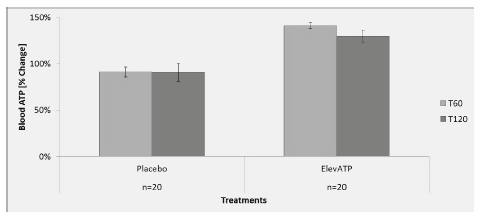
Figure 1: Effect of elevATP™ on blood ATP levels. elevATP™ significantly increased blood ATP levels by 40% at T60 (p
A single dose of 150 mg elevATP significantly increased blood ATP by 40% at 60 minutes (p
Plasma ATP levels were measured using 10 µL of plasma in a luciferase-based assay. There was no significant increase in ATP level at T60 (p=0.83) or T120 (p=0.69) in patients treated with elevATP™ (Figure 2). No change in plasma ATP level was seen after treatment with placebo.
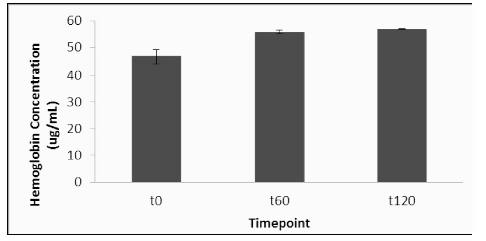
Hemoglobin levels were determined in all plasma samples in order to ensure that mechanical disruption of erythrocytes did not affect plasma ATP levels. The placebo group had an increase in plasma hemoglobin of 34% at T60 and 37% at T120 on day 1, compared to the T0 baseline (Figure 3). On day 2, after treatment with elevATP™, there was an increase in plasma hemoglobin level of 4% at T60 and 28% at T120, compared to the new T0 baseline. There were no statistically significant differences in placebo and elevATP™ treatments at T60 (p=0.29) and T120 (p=0.76).
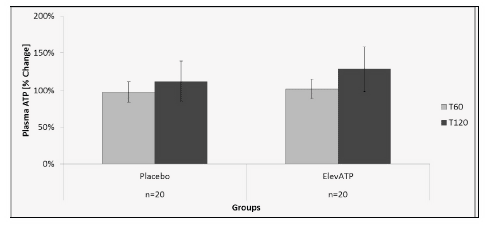
Figure 2: Plasma ATP levels after treatment with elevATP™. Data is presented as Mean +/- SE, Data are presented as % change over baseline T0. n=20.
Blood glucose levels were monitored after treatment with placebo (Day 1) and after elevATP™ (Day 2), as previously described. There were no significant differences in blood glucose levels between treatments at T60 (p=0.57) or T120 (p=0.59) in the 20 patients examined. Blood lactate levels remained unchanged after placebo (Day 1) or elevATP™ (Day 2) administration. The difference between treatments was not significant either at T60 (p=0.61) or T120 (p=0.44).
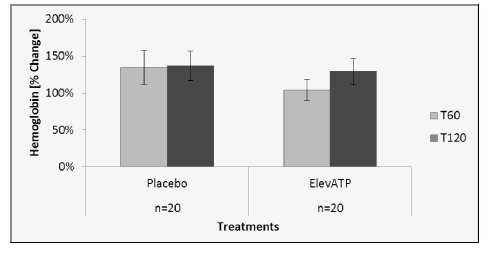
Figure 3: Hemoglobin levels after treatment with elevATP™. There was a 4% increase at T60 and 28% increase at T120. There were no statistical differences when compared to placebo. Data are presented as Mean +/- SE, n=20.
Biceps muscle levels of ATP were determined before and after administration of elevATP™. At T0 (prior to treatment), 210 pg ATP per mg protein was detected (Figure 4). ATP level increased in muscle biopsy tissue to 590 pg/mg protein at 60 minutes and 910 pg/mg protein at 120 minutes. Hemoglobin was also quantified in muscle biopsy lysates in order to ensure that mechanical disruption of the tissue did not affect ATP levels (Figure 5). Hemoglobin levels did not increase after treatment.
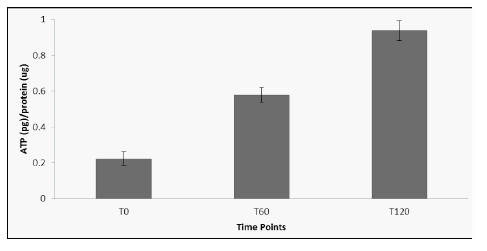
Figure 4: ATP levels in muscle tissue after treatment with elevATP™. ATP levels increased significantly after treatment. Data is presented as mean +/- SE of 4 determinations.
Discussion
These findings provide further support for the assertion that oral administration of elevATP™ increases whole blood levels of ATP in healthy humans. The increases reported here are in agreement with our previous report (16). elevATP™ appears to selectively and acutely increase ATP levels within the cellular component of blood. In contrast, ATP levels in cell-free plasma remained unchanged following elevATP™ ingestion. We verified the integrity of erythrocyte cell membranes by quantifying hemoglobin concentrations in plasma. This suggests that ATP did not originate from ruptured red blood cells. Likewise, we found no changes in plasma ATP levels after treatment with elevATP™, suggesting that it is unlikely that elevATP™ affects extracellular ATP levels.
Red blood cells constitute the largest cellular component of blood, comprising approximately 40 to 50% of the blood volume. Red blood cells are a major carrier of ATP (19-21), with intracellular concentrations reaching the millimolar range (17, 22). ATP production in erythrocytes takes place via glycolysis, rather than within mitochondria (19). We previously reported that ingestion of elevATP™ by fasting and resting study subjects did not alter blood lactate levels, which could be generated by increased glycolysis and ATP production in red cells under these experimental conditions (16). These results suggest that red blood cells did not contribute to elevation of whole blood ATP level.
Fresh whole blood was collected and immediately lysed to measure total ATP. This included ATP originating from all types of cells present in the blood collected. The lack of changes in blood glucose and lactate levels suggests that ATP originated from blood cells with mitochondria, such as platelets and while blood cells. Platelets, unlike red blood cells, do contain mitochondria. Platelets contain roughly 40 nmol of ATP and ADP per mg of platelet protein (23). Platelets can be stimulated through thrombin activation to achieve low micromolar concentrations of ATP and ADP (24).
Further support for increased intracellular organ ATP formation came from our muscle biopsy case study. As presented here, muscle tissue, which is rich in mitochondria, exhibited a substantial increase in ATP levels after elevATP™ ingestion. Additional work in a larger number of volunteers is in preparation to confirm the stimulatory effect of elevATP™ on intramuscular ATP levels in resting subjects. Our previous work showed that elevATP™ did not increase reactive oxygen species, despite increasing ATP levels (16). The relationship between elevATP™ and mitochondrial Complex IV and/or V activity is currently under investigation in our laboratory as well as establishing ATP/phosphocreatine ration before and after ingestion of elevATP
In conclusion, we have thus confirmed the ability of elevATP™ to increase ATP levels in whole blood. We have further shown that elevATP does not increase ATP levels in plasma. The precise blood cell type or types that are sensitive to the action of elevATP™ is not known. A clinical case experiment demonstrated that ingestion of a single dose of elevATP™ resulted in a significant increase in intramuscular ATP under resting conditions. This result suggests that ingestion of elevATP™ may increase ATP level in other tissues. Further clinical testing is justified and is needed to confirm this preliminary result. Investigating whether elevATP™ may improve muscle performance and endurance in young and aged individuals and such experimentation is also justified and is currently in preparation.
Acknowledgments: All authors declare that they have no conflicts of interest. The present study was funded by Futureceuticals, Inc. We express our gratitude to John Hunter and Brad Evers (FutureCeuticals, Inc.) for their comments and suggestions in the preparation of this article. We would like to thank Michael Sapko for his Paroxetine help in editing the manuscript.
References
-
Gordon JL. Extracellular ATP: effects, sources and fate. Biochem J 1986;233:309-319
-
Trautmann A. Extracellular ATP in the immune system: more than just a “danger signal”. Sci Signal 2009;2:pe6 doi 10.1126/scisignal.256pe6
-
Haag F, Adriouch S, Brass A, Jung C, Moller S, Scheuplein F, Bannas P, Seman M, Koch-Nolte F. Citalopram Extracellular NAD and ATP: Partners in immune cell modulation. Purinergic Signal 2007;3:71-81 doi 10.1007/s11302-006-9038- 7
-
Miyazawa M, Ito Y, Kosaka N, Nukada Y, Sakaguchi H, Suzuki H, Nishiyama N. Role of TNF-alpha and extracellular ATP in THP-1 cell activation following allergen exposure. J Toxicol Sci 2008;33:71-83
-
Schwiebert EMZ, A. Extracellular ATP as a signaling molecule for epithelial cells. Biochim Biophys Acta 2003;1615:7-32 doi 10.1007/s11302-005-0777-7
-
Jordan ANJ, R.; Abraham, E. H.; Salikhova, A.; Mann, J. K.; Morss, G. M.; Church, T. S.; Lucia, A.; Earnest, C. P. Effects of oral ATP supplementation on anaerobic power and muscular strength. Med Sci Sports Exerc 2004;36:983- 990
-
Miyoshi NO, H.; Chock, P. B.; Stadtman, E. R. Age-dependent cell death and the role of ATP in hydrogen peroxide-induced apoptosis and necrosis. Proc Natl Acad Sci U S A 2006;103:1727-1731 doi 10.1073/pnas. 0510346103
-
Subasinghe W, Spence DM. Simultaneous determination of cell aging and ATP release from erythrocytes and its implications in type 2 diabetes. Anal Chim Acta 2008;618:227-233
-
Kirby BS, Crecelius AR, Voyles WF, Dinenno FA. Impaired skeletal muscle blood flow control with advancing age in humans: attenuated ATP release and local vasodilation during erythrocyte deoxygenation. Circ Res 2012;111:220-230 doi 10.1161/circresaha.112.269571
-
Rathmacher JA, Fuller JC, Jr., Baier SM, Abumrad NN, Angus HF, Sharp RL. Adenosine-5′-triphosphate (ATP) supplementation improves low peak muscle torque and torque fatigue during repeated high intensity exercise sets. J Int Soc Sports Nutr 2012;9:48 doi 10.1186/1550-2783-9-48
-
Jordan AN, Jurca R, Abraham EH, Salikhova A, Mann JK, Morss GM, Church TS, Lucia A, Earnest CP. Effects of oral ATP supplementation on anaerobic power and muscular strength. Med Sci Sports Exerc 2004;36:983-990
-
Arts IC, Coolen EJ, Bours MJ, Huyghebaert N, Stuart MA, Bast A, Dagnelie PC. Adenosine 5′-triphosphate (ATP) supplements are not orally bioavailable: a randomized, placebo-controlled cross-over trial in healthy humans. J Int Soc Sports Nutr 2012;9:16 doi 10.1186/1550-2783-9-16
-
Kichenin K, Decollogne S, Angignard J, Seman M. Cardiovascular and pulmonary response to oral administration of ATP in rabbits. J Appl Physiol 2000;88:1962-1968
-
Agteresch HJ, Dagnelie PC, Rietveld T, van den Berg JW, Danser AH, Wilson JH. Pharmacokinetics of intravenous ATP in cancer patients. Eur J Clin Pharmacol 2000;56:49-55
-
Pastor-Anglada M, Errasti-Murugarren E, Aymerich I, Casado FJ. Concentrative nucleoside transporters (CNTs) in epithelia: from absorption to cell signaling. J Physiol Biochem 2007;63:97-110
-
Reyes-Izquierdo T, Nemzer B, Argumedo R, Shu C, Huynh L, Pietrzkowski Z. Effect of the dietary supplement ElevATP on blood ATP level: An acute pilot clinical study. J Aging Res Clin Practice 2013;2:178-184
-
Gorman MW, Feigl EO, Buffington CW. Human plasma ATP concentration. Clin Chem 2007;53:318-325
-
Reyes-Izquierdo TH, L. E.; Sikorski, R.S.; Nemzer, B.; Pietrzkowski, Z. Acute effect of HH2o on oxygen consumption rate, intracellular ATP and ROS in freshly isolated human peripheral blood mononuclear cells. Current Topics in Nutraceutical Research 2011;9:139-146
-
Ellsworth ML, Forrester T, Ellis CG, Dietrich HH. The erythrocyte as a regulator of vascular tone. Am J Physiol 1995;269:H2155-2161
-
Sprague RS, Ellsworth ML, Stephenson AH, Kleinhenz ME, Lonigro AJ. Deformation-induced ATP release from red blood cells requires CFTR activity. Am J Physiol 1998;275:H1726-1732
-
Sprague RS, Hanson MS, Achilleus D, Bowles EA, Stephenson AH, Sridharan M, Adderley S, Procknow J, Ellsworth ML. Rabbit erythrocytes release ATP and dilate skeletal muscle arterioles in the presence of reduced oxygen tension. Pharmacol Rep 2009;61:183-190
-
Montalbetti N, Leal Denis MF, Pignataro OP, Kobatake E, Lazarowski ER, Schwarzbaum PJ. Homeostasis of extracellular ATP in human erythrocytes. J Biol Chem 2011;286:38397-38407 doi 10.1074/jbc.M111.221713
-
Da Prada M, Richards J, Kettler R. Amine storage organelles in platelets. Platelets in biology and pathology 1981;2:107-145
-
Ingerman CM, Smith JB, Silver MJ. Direct measurement of platelet secretion in whole blood. Thrombosis research 1979;16:335-344